Clinical Efficacy and Complications of Intraocular Lens Exchange for Opacified Intraocular Lenses
Article information
Abstract
Purpose
To evaluate the clinical efficacy and complications of intraocular lens (IOL) exchange.
Methods
A review of medical records was performed for 52 eyes that had undergone an IOL exchange due to IOL opacification. Surgical complications and their incidences were analyzed. The mean best corrected visual acuity (BCVA) after the IOL exchange was compared with the mean pre-exchange BCVA and with the mean BCVA after the initial IOL implantation. Prediction error of refraction and biometric data obtained for the IOL exchange were, if available, compared with those obtained for the initial IOL implantation. The prediction error for the IOL exchange, calculated from the biometric data obtained before the IOL exchange, was compared with that calculated from the measurements obtained before the initial IOL implantation.
Results
The overall complication rates were low and no serious complications were found. The mean BCVA improved significantly after the IOL exchange and was not significantly different from that obtained after the initial IOL implantation. However, the refractive prediction for the IOL exchange was not as good as it was for the initial IOL implantation, which was thought to be related with difficulties in axial length (AL) measurements. Biometric data taken before the initial IOL implantation was associated with a significantly better refractive prediction than those taken before the IOL exchange.
Conclusions
IOL exchange was both efficacious and safe for visual recovery. However, IOL exchange was related with increased difficulty of predicting postoperative refraction; difficulties in AL measurements are the suggested cause.
Implantation of a foldable intraocular lens (IOL) takes an essential part of modern cataract surgery. Since SI-18NB (AMO®, USA) was used as the first foldable IOL,1 there have been many changes made in IOL shape and composition to improve surgical outcomes and to minimize complications. Despite the remarkable improvements in the urrently used IOLs, there have been discouraging clinical reports regarding unexpected late opacification of the implanted IOLs which is of concern to both patients and surgeons. Delayed opacification of foldable hydrophilic acrylic IOLs is a well-known phenomenon. Pathological studies using light microscopy, scanning electron microscopy, and X-ray spectroscopy have revealed that the opacification was caused by granular deposits composed of calcium phosphate hydroxide (hydroxyapatite) under the surface of the IOL.2-4 Neodymium:YAG (Nd:YAG) laser treatment was used to manage this complication by some authors.5 Although the authors attempted to blast the deposits from the IOL with laser treatment, the results were disappointing. Nd:YAG laser treatment did not remove the deposits or improve vision. Meanwhile, several studies have reported that an IOL exchange improves vision in such cases.5-8
In addition to IOL opacification, there are several circumstances under which explantation of an IOL, with or without implantation of a new IOL, is required as the definitive treatment. These circumstances include implantation of an IOL with incorrect power, an IOL defect, abnormal IOL position, glare symptoms, uveitis glaucoma hyphema (UGH) syndrome, and chronic uveitis.9-11 However, it seems that the indications for an IOL exchange are not limited to the management of these complicated cases, but may also include those patients requiring refractive changes. There are already several reports regarding IOL exchange procedures performed for patients favoring monovision in whom the initial IOL implantation surgeries resulted in similar refractive outcome in both eyes and for those who experienced unexpected hyperopic or myopic surprises.9,12 With further popularization of multifocal IOLs, it seems that patients who have already undergone a monofocal IOL implantation surgery may also be benefited from an IOL exchange procedure.
The principal purpose of this study is to evaluate the clinical outcome of IOL exchange, including its efficacy in visual recovery and its complications. This study also provides some insight into the determination of adequate IOL power for IOL exchange performed for the eyes with an opacified IOL.
Materials and Methods
The present study includes consecutive cases of 52 eyes from 48 patients treated with IOL exchange at the Korean Veterans hospital between January 2005 and December 2006.
Before the IOL exchange surgery, IOL power was calculated using the keratometric value and the axial length (AL) obtained either before the initial cataract surgery or before the IOL exchange. In 32 cases, reevaluation of biometric values was performed using an autokeratorefractometer (Humphrey-Zeiss model 599, USA) and an applanation ultrasound (US) A-scanner (Paradigm Medical Industries model p37, USA) using the mean pseudophakic US velocity of 1533 m/sec in pseudophakic mode. All of the IOL measurements for initial IOL implantation taken in our hospital were performed by one technician and all of the repeat IOL measurements before the IOL exchange were done by another technician. The SRK/T formula was used to calculate IOL power.
All IOL exchange surgeries were performed by one experienced surgeon. Retrobulbar anesthesia using 2% lidocaine was used in all cases. After a 1.2 mm-sized clear corneal paracentesis incision was made at the temporal side, a superior scleral tunnel incision was made with a width of approximately 6 mm. The ophthalmic viscoelastic device was then introduced into the anterior chamber. A 23 gauge needle with a bent tip was used to relieve adhesion between the lens capsule and the opacified IOL. Usually there were firm adhesions between the lens capsule and the IOL, and care was taken not to exert excessive force. Viscodissection was carefully applied to further relieve the adhesions and to safely separate the IOL haptics from the lens capsule. In some cases in which there were excessively strong adhesions, one or both of the haptics had to be cut with Vannas scissors and left in the capsular bag to avoid damage to the lens capsules and/or the zonules.5,13 The optic was also sometimes bisected before removal. After safely removing the opacified IOL, a new foldable posterior chamber IOL was inserted either into the capsular bag or into the ciliary sulcus. In all cases in which the size of the capsulorhexis opening was smaller than the optic size, optic capture with the anterior capsule was attempted. Two types of IOLs were used for the exchange procedures: Sensar® (Advanced Medical Optics, USA) and Acrysof® (MA60BM)(Alcon, USA). Finally, the viscoelastic device was removed by irrigation and aspiration and the scleral wound was closed with interrupted 10-0 nylon sutures.
During postoperative follow-up, visual acuity (VA), refraction, and intraocular pressure (IOP) were monitored. VAs were evaluated with a standard Snellen chart and converted to logMAR for statistical analysis. VA that could barely detect a hand movement was defined as 0.001 in decimal scale. Refractions were measured by an autokeratorefractometer (Humphrey-Zeiss model 599, USA), confirmed by subjective refractions, and converted to spherical equivalents (SEs) for statistical analysis. Postoperative best corrected visual acuity (BCVA) and refraction were obtained at least 1 month after each operation. The IOP was considered to be elevated when it exceeded 21 mmHg or was increased by more than 5 mmHg with regard to its preoperative level.
Data collected included age, gender, presence of systemic (diabetes mellitus and hypertension) and/or ophthalmic comorbidities, interval between initial IOL implantation and IOL exchange, site of IOL implantation during the IOL exchange, intraoperative complications and execution of anterior vitrectomy during the IOL exchange procedure, length of postoperative follow-up after the IOL exchange, pre- and post-exchange VAs and IOPs, and execution of an Nd:YAG posterior capsulotomy after the IOL exchange.
The following data were also retrieved when available: Type of initial cataract surgery, power and material of the initially implanted IOL, site of IOL implantation, biometric data used for the IOL calculation (both for the initial and the exchange procedures), IOL calculation formula, and the BCVA and refractions after the initial IOL implantation.
We analyzed the complications related to the IOL exchange and their incidences. The mean BCVA obtained after IOL exchange was compared with that recorded before IOL exchange and, when available, also with the mean BCVA obtained after the initial IOL implantation surgery.
For 32 cases in which the biometric data obtained both before the initial IOL implantation surgery and before the IOL exchange were available, further analysis was conducted regarding the predictability of postoperative refraction. The prediction error of postoperative refraction was defined as the actual obtained postoperative SE minus the intended SE given the implanted IOL power, and its absolute value was defined as the absolute error. Cases included in this analysis were grouped according to the site of IOL implantation during IOL exchange; there was an in-the-bag implantation group and an in-the-sulcus group. The refractive results were compared between these two groups. Within each group, the prediction error for the IOL exchange was then compared with the prediction error for the initial IOL implantation surgery. The reevaluated keratometric value and AL were also compared with the values obtained before the initial IOL implantation surgery. The prediction errors of refraction which were calculated using these two biometric datasets were compared.
Statistical analyses were performed using Microsoft Excel and SPSS (version 12.0, SPSS Inc, Chicago, IL). Results were considered statistically significant only when the p-value was less than 0.05.
Results
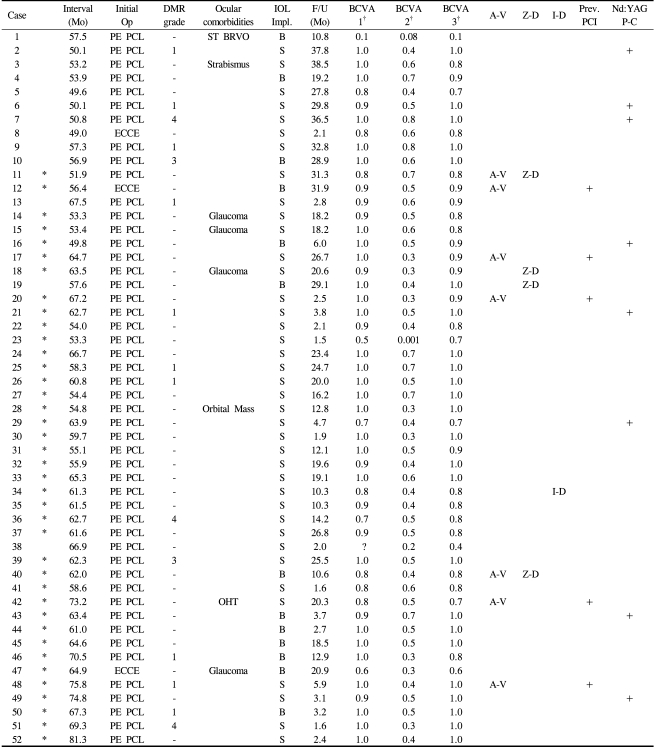
Table 1 presents a summary of the total cases. The subjects included were 36 men (75%) and 12 women (25%); the mean age of the patients was 70.1±7.8 years (range: 49-84). Four of the male patients had undergone bilateral IOL exchanges. Fourteen patients (29.2%) were diabetic and 23 patients (47.9%) had systemic hypertension. Fifteen eyes (31.3%) were from diabetic patients, of which 10 (19.2%) had no diabetic retinopathy, 2 (3.9%) had mild nonproliferative diabetic retinopathy, and 3 (5.8%) had moderate nonproliferative diabetic retinopathy according to the early treatment of diabetic retinopathy study (ETDRS) criteria.14 Other ocular comorbidities included glaucoma in 4 eyes (7.7%), a preexisting superotemporal branch retinal vein occlusion in 1 eye (1.9%), and a magnetic resonance imaging-proven orbital mass encircling the optic nerve in 1 eye (1.9%).
The initial procedure was a phacoemulsification surgery in 49 eyes and an extracapsular cataract extraction in 3 eyes. The mean time interval between initial IOL implantation and the IOL exchange was 60.4±7.4 months (range: 49.0-81.3) and the mean length of follow-up after IOL exchange was 15.6±11.4 months (range: 1.5-38.5).
During IOL exchange, a new IOL was implanted in the bag in 13 eyes (25.0%) and in the sulcus in 39 eyes (75.0%) with or without optic capture. Zonular dehiscence occurred in 4 eyes (7.7%) and iridodialysis in 1 eye (1.9%). Preexisting posterior capsular incompetency was observed in 4 eyes (7.7%). Seven eyes (13.5%) were treated with concomitant anterior vitrectomy. During postoperative follow-up, 8 eyes (15.4%) were treated with a Nd:YAG posterior capsulotomy because of posterior capsular opacification. One eye (case 19) that had developed a delayed IOL dislocation associated with severe capsular contracture was treated with IOL repositioning by suture fixation to the sclera. Aside from case 19, no eye developed serious complications such as endophthalmitis, intractable corneal decompensation, or retinal detachment. IOP elevation was observed in 13 eyes (25%) after 1 week, in 5 eyes (9.6%)after 1 month, and was normalized in all cases at the last outpatient visit.
The best recorded BCVA after initial IOL implantation was available in 51 cases (case 38 was the exception). The mean BCVA was 0.90±0.16 (0.06±0.15 logMAR) after initial IOL implantation, 0.48±0.16 (0.39±0.41 logMAR) before IOL exchange, and 0.88±0.16 (0.07±0.15 logMAR) after IOL exchange. A paired t-test revealed that the mean BCVA before IOL exchange improved significantly after IOL exchange (p<0.001) and it was not significantly different from the mean BCVA obtained after the initial IOL implantation (p=0.130). There was an increase of nearly 4 lines in the mean BCVA after IOL exchange.
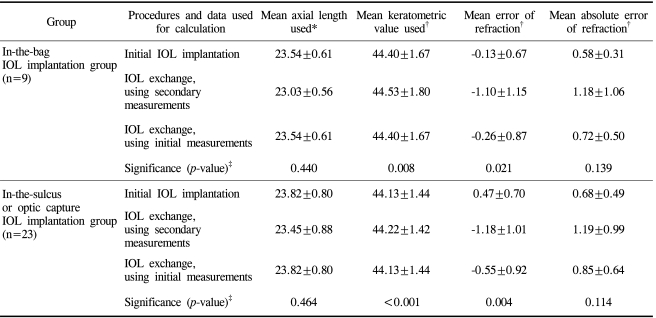
Table 2 summarizes the results of the analysis on the prediction error of postoperative refraction for the IOL exchange. In 32 eyes (cases marked with an asterisk (*)), the keratometric value and the AL were determined both before the initial IOL implantation and before the IOL exchange. The mean keratometric values before initial IOL implantation and before IOL exchange were 44.20±1.48 diopters (D) and 44.30±1.51 D, respectively, while the mean ALs were 23.74±0.75 mm and 23.30±0.82 mm, respectively. A paired t-test revealed that the difference between ALs was statistically significant (p<0.001), while the difference in the mean keratometric values was not significant (p=0.222). A mean shortening of the ALs of about 0.43 mm was observed before the IOL exchange.
In the group with new IOL implantation in the bag (9 cases), the mean prediction error and the mean absolute error for the initial IOL implantation were -0.04±0.66 D and 0.56±0.30 D, respectively. The mean prediction error and the mean absolute error for the IOL exchange calculated from the measurement data taken before the IOL exchange were -1.10±1.15 D and 1.18±1.06 D, respectively. The mean prediction error and the mean absolute error for the IOL exchange using the data taken before the initial IOL implantation were -0.26±0.87 and 0.72±0.50 D, respectively. The two biometric data resulted in significantly different mean prediction errors (p=0.021), indicating significantly better refractive prediction with biometric data obtained before the initial IOL implantation. The mean ALs before the initial IOL implantation and before the IOL exchange were 23.54±0.61 mm and 23.03±0.56 mm, respectively. The mean keratometric values before the initial IOL implantation and before the IOL exchange were 44.40±1.67 D and 44.53±1.80 D, respectively. A Wilcoxon signed rank test showed that the difference in mean AL measurements was statistically significant (p=0.008), while the difference in mean keratometric values was not significant (p=0.440).
For the in-the-sulcus group (23 cases), the mean error and the mean absolute error were 0.49±0.72 D and 0.70±0.50 D for the initial IOL implantation, -1.18±1.01 D and 1.19±0.99 D for the IOL exchange using the data taken before the IOL exchange, and -0.55±0.92 D and 0.85±0.64 D for the IOL exchange using the data taken before the initial IOL implantation, respectively. These two biometric data resulted in a significantly different mean prediction error for the IOL exchange (p=0.004). A better refractive prediction was associated with the biometric data obtained before the initial IOL implantation. The mean ALs measured before the initial IOL implantation and before the IOL exchange were 23.82±0.80 mm and 23.45±0.88 mm, respectively, while the mean keratometric values were 44.13±1.44 D and 44.22±1.42 D, respectively. A Wilcoxon signed rank test showed that the difference in mean AL was significant (p<0.001). In contrast, the difference in mean keratometric values was insignificant (p=0.464).
Discussion
There have been a number of clinical reports regarding the phenomenon of late postoperative opacification of hydrophilic acrylic IOLs.1-6,15-21 The involved IOLs included the Hydroview® (Bausch & Lomb) and the MemoryLens® (Mentor Ophthalmics, Inc.), which had calcified deposits mostly on the optic surface, and the Aqua-sense® (Ophthalmic Innovations International, Inc.), which had calcified deposits mostly within the IOL material. Furthermore, reports have been published in the Republic of Korea regarding late opacification of a hydrophilic acrylic IOL, the ACRL-C160® (Ophthalmed, USA).1-4 In such cases, an IOL exchange procedure may be the definitive treatment modality. However, there have been only a few reports regarding the surgical outcomes of IOL exchange.5,13,22,23 The principal purpose of this study was to review the clinical results, including the efficacy and complications, of IOL exchange due to an opacified IOL.
Some authors4 have suggested that the pathogenic mechanism for late opacification of a hydrophilic acrylic IOL might be related to a systemic morbidity, such as diabetes mellitus. However, in our study, opacification of the IOL was readily observed in 37 eyes (71.2%) from non-diabetic subjects.
In our study, the time interval between the initial cataract surgery and the IOL exchange was 60.4±7.4 months. A previous study1 reported that the development of hydrophilic acrylic IOL opacification was observed after 7.8±3.0 months in patients with diabetes mellitus and after 14.9±5.8 months in those without diabetes. The large discrepancy between the previously reported time interval for initiation of IOL opacification and the time interval for IOL exchange surgery in our study can be explained by the fact that there may have been individual differences in the rate of progression of opacification and that some patients might have had useful vision despite opacification of their IOL. We believe that the decision to undergo a second surgery should be made deliberately and must take into account all potential risks and benefits.
The intraoperative complication rates in our series were at least comparable to or relatively lower than those demonstrated by previous reports. Yu et al.5 reported 2 posterior capsule (PC) ruptures and 3 zonular dehiscences in a series of 15 explantation surgeries for calcified IOLs. Dagres et al.22 reported 1 case of PC rupture (4%), 10 cases of zonular dehiscence (40%), and 1 case with PC rupture and zonular dehiscence (4%) in 25 eyes. Gashau et al.13 reported a 23.1% occurrence rate of vitreous loss necessitating anterior vitrectomy. We observed zonular dehiscence in 4 eyes (7.7%) and iridodialysis in 1 eye (1.9%). Anterior vitrectomy was performed in 7 eyes (13.5%). The lack of vision-threatening complications in our study also demonstrates that the IOL exchange procedures were performed with sufficient safety.
In our study, the mean BCVA after IOL exchange was 0.88±0.16 (0.07±0.15 logMAR) and the improvement in BCVA was statistically significant (p<0.001) with regard to the mean BCVA before IOL exchange, which was 0.48±0.16 (0.39±0.41 logMAR). Published reports on the mean BCVA before and after IOL exchange vary according to the surgeons. Dagres et al.22 reported a BCVA of 0.57±0.24 (decimal scale) preoperatively and 0.60±0.28 (decimal scale) postoperatively at the last follow-up visit. They also mentioned that some of the eyes (25%) in their study were awaiting Nd:YAG posterior capsulotomy for severe posterior capsular opacity at the time of publication. Yu et al.5 reported that the mean BCVA 3 months after the exchange procedure was 0.2, which was similar to the VA level (0.25) measured 3 months after the initial IOL implantation. They explained the lower post-exchange VA by mentioning that their cases had probably included a high percentage of patients with diabetic retinopathy and that the IOL exchange procedures were performed primarily to allow more clear visualization of their retinas. Jin et al.9 reported that for posterior chamber IOL, the mean pre-exchange BCVA was 0.13±0.15 logMAR and the mean post-IOL exchange BCVA was 0.06±0.12 logMAR. Their series included various indications for IOL exchange and was not confined to the management of opacified IOLs.
It would be reasonable to state that the efficacy of IOL exchange can be better appreciated by comparing the post-exchange BCVA with the best BCVA obtained after the initial IOL implantation, rather than by comparing the BCVA measured just before the IOL exchange, at which time VA would be affected by variable degrees of IOL opacification. Our data clearly demonstrated that the mean BCVA after IOL exchange was not statistically different from the mean best BCVA obtained after initial IOL implantation. Our data also showed none had decrease in VA after IOL exchange with regard to pre-exchange VA. These indicate that the IOL exchange procedure was not only efficacious in restoring VA, but was also safe and tolerable. The type and nature of the IOL initially implanted, the type of the initial cataract surgery and its complications, the surgical techniques used in the IOL exchange, and the experience of the surgeon may be related to variable surgical outcomes.
Although we obtained good results with regard to visual recovery, the IOL exchange procedure was generally associated with a slightly increased difficulty in predicting postoperative refraction.
The refractive predictability of the IOL exchange was relatively good when a secondary IOL was implanted in the bag and when measurement values taken from the initial IOL implantation were used to calculate the IOL power. The minimal difference between the mean prediction error of -0.13±0.67 D after the initial IOL implantation and -0.26±0.87 D taken after the IOL exchange for the in-the-bag group suggests that the refractive prediction of IOL exchange may not be greatly affected if measurement data obtained before the initial IOL implantation were of good quality and readily available. However, the mean prediction error increased to -1.10±1.15 D when the biometric data obtained just before the IOL exchange were used.
Compared to the in-the-bag group, there was a slightly larger myopic shift in the mean postoperative refraction for the in-the-sulcus group. The mean prediction error of 0.49±0.70 D obtained after the initial IOL implantation and -0.55±0.92 D obtained after the IOL exchange using the same biometric data taken before the initial IOL implantation imply that a mean refractive shift of about -1.04 D occurred. This shift can be partially explained by the more anterior positioning of the new IOLs with regard to the capsular bag.24,25 In our study, the power of the new IOL was adjusted by an amount ranging from -0.5 to -1.0 D if the IOL was to be implanted in the sulcus. Also, for the in-the-sulcus group, the biometric data obtained just before the IOL exchange were associated with increased prediction errors resulting in a mean prediction error of -1.18 D, indicating additional deterioration of the mean postoperative refraction by -0.63 D. Regardless of the site of IOL implantation for the IOL exchange, the biometric data taken just before the IOL exchange were associated with increased prediction errors of refraction.
Statistical analyses comparing the biometric data obtained before the initial IOL implantation surgery and those obtained just before the IOL exchange revealed that there was no significant difference in the mean keratometric values, but there was a significant difference in the mean ALs. This suggests that the keratometric change, which was assumed to be induced by the initial cataract surgery, was actually negligible and the change in the ALs was the major factor responsible for the decreased refractive predictability. In our study, the mean AL measured just before the IOL exchange was approximately 0.3-0.4 mm shorter; this was thought to be related to difficulties in taking AL measurements using an US A-scan in eyes with opacified IOLs.
Kora et al.26 reported that mean corneal refractive powers were not statistically different before and after cataract surgery, but that the ALs were markedly different in some cases. They noted that it was difficult to determine the appropriate AL for the IOL exchange. They calculated the ALs retrospectively using the Holladay or SRK/T formula, and reported that the calculated ALs were much longer than the values that were measured directly. Many eyes in their series had become myopic after the initial IOL implantation, which implies that the initially measured ALs might have been slightly shorter. The authors concluded that even after correcting the AL and A-constant, they could not achieve precise refractive prediction for the IOL exchange. They suggested that other factors probably contributed to the increased error in IOL power determination.
The US AL biometry measures the time needed for an ultrasonic wave to travel through the cornea, anterior chamber, lens, and vitreous. To calculate the distance that the ultrasound traveled, the velocity must be known. Different ocular components have different US velocities, so accurate measurement of the AL requires that US velocities are properly assumed for these tissues. Table 3 shows the US velocities used by several authors.27-30 According to Holladay and Prager,31,32 the actual pseudophakic AL can be expressed using the following equation:
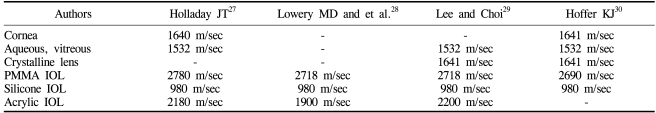
US velocities used by various authors for various ocular tissues and IOL materials at body temperature (35-37℃)
AL = ALM1532 + LT(1-1532/VIOL) = ALM1532 + CALF
where AL=actual pseudophakic AL, ALM1532=AL measured at 1532 m/sec, LT=center thickness of the IOL, VIOL=US velocity of the IOL material, and CALF=corrected AL factor. The center thickness of an IOL is variable according to the manufacturer's design and the diopteric powers, both of which are not always available to the surgeon performing the IOL exchange. Assuming an acrylic IOL with an US velocity of 2180 m/sec and a central thickness of 0.7-1.0 mm, a CALF of 0.207-0.297 mm is obtained. An US velocity of 2200 m/sec, as used by Lee and Choi,29 yields a CALF of 0.212-0.303 mm. This potential variability in the CALF may partly explain the mean shortening of the AL observed just before IOL exchange. A limitation of this approach is that the material and/or central thickness of an opacified IOL are not always available in clinical practice. There are also concerns regarding the extent to which the AL measurement may be affected by various degrees of opacification.
The standard deviations (intercase variability) of the prediction errors were generally greater in the IOL exchange group compared with the initial IOL implantation group and were slightly larger for the in-the-sulcus group than for the in-the-bag group. Altered optical properties of the opacified IOLs and a US velocity that was not adequate for IOLs with various degrees of opacification may be related to the increase in the mean and standard deviation of the prediction errors. Operator-related errors could have also been responsible, because each pair of AL measurements was performed by different technicians. Increased variability in the actual postoperative positioning of the IOL with regard to the initial IOL implantation surgery may also have been responsible. Jin et al.9 reported their experience with IOL exchange due to incorrect lens power and suggested that the refractive surprise after cataract surgery could be attributed to inherent errors in the biometry and formula, which may not be appropriate for certain eyes. Their observation may also provide some explanation for the increased prediction errors related to IOL exchange in our study. However, our data suggest that if the biometric data obtained before IOL exchange are available and of good quality, IOL exchange with implantation of a new IOL in a more stable position (in-the-bag position) may be associated with a sufficiently small amount of prediction error of refraction.
To our knowledge, this is the first clinical report from the Republic of Korea regarding the clinical efficacy and complications of the IOL exchange procedure for an opacified IOL. Our data showed excellent visual recovery results and good results with regard to complications. Although the predictability of the postoperative refraction of the IOL exchange was relatively good using biometric data obtained before the initial cataract surgery and employing a new IOL implantation in-the-bag, we faced difficulties with the refractive predictions calculated using biometric measurements obtained just before the IOL exchange. Difficulties in making the AL measurement in eyes with an opacified IOL was the most probable cause. Although our study was limited by a relatively small number of cases, our experience suggests that good quality initial biometric dataand new IOL implantation in the bag can produce good postoperative refractive results. Use of an US velocity appropriate for the IOL is also likely to result in improved refractive prediction. We believe that an IOL exchange procedure should be performed only after comprehensive informed consent is given by the patient. Dagres et al.22 mentioned that various symptoms and complaints reported by patients with opacified IOLs did not correlate well with their VAs and that VA alone was not a good criterion for performing an IOL exchange. The surgeon must be deliberate in making decisions regarding whether or not an IOL exchange surgery should be performed for an eye with an opacified IOL.
IOL exchange surgery is the only effective treatment for patients with an opacified IOL. However, indications for IOL exchange are no longer limited to complications following IOL implantation and they are becoming even larger due to the increasing refractive demands of patients previously treated with IOL implantation. It would be worthwhile for ophthalmologists to become familiar with the procedures, efficacies, limitations, and complications associated with IOL exchange. We hope that further studies with a larger group of patients and proper stratification may help provide solutions to the problems that we encountered with IOL exchange.