A Comparison of the Efficacy of Cataract Surgery Using Aqualase® with Phacoemulsification Using MicroFlow® System
Article information
Abstract
Purpose
To compare the outcomes after phacoemulsification performed with the AquaLase® and phacoemulsification in MicroFlow® system, including surgically induced astigmatism (SIA), corneal endothelial cell damage and postoperative recovery of visual acuity.
Methods
The cataracts of Lens Opacities Classification System, version III (LOCS III) nuclear grade below 2 were subjected in this study. Nineteen eyes underwent cataract operation using AquaLase® (Alcon Laboratories, Fort Worth, Texas, U.S.A.). A control group (19 eyes) used the MicroFlow® system (Millenium, Stortz, U.S.A.) and was selected by matching age, sex, systemic disease, corneal astigmatism and corneal endothelial cell density. All the surgeries were performed by the same operator. SIA, corneal endothelial cell loss, visual acuity, and corneal thickness were evaluated postoperatively.
Results
SIA in the group using AquaLase® was less than that of the group using MicroFlow® system (P=0.022) at 2 months postoperatively. Evaluation of corneal endothelial cell loss, recovery of visual acuity and corneal thickness found no statistically significant differences between the two groups.
Conclusions
Cataract surgery using AquaLase® induces less surgically induced astigmatism in mild to moderate cataracts.
Since Kelman first introduced phacoemulsification in 1967, there have been numerous developments in the removal of the cataracts. Recently introduced, AquaLase® is one of the methods in which to remove a cataract using the Infinity Vision System (Alcon Laboratories, Fort Worth, Texas). It uses water-jet pulses to liquefy the lens nucleus. The AquaLase® handpiece warms a balanced salt solution up to 57℃ and then creates a micropulse (4 µl, 50 pulses per second) for extracting the cataract.1
The theoretically proposed advantages of AquaLase® for removing a cataract are reduced thermal risk (no incisional burn) and reduced posterior capsular rupture.1,2
The MicroFlow® system was developed by Bausch & Lomb Surgical with a grooved outer surface that allows for an increased rate of fluid flow into the eye, keeping the needle cool. The MicroFlow® system delivers improved performance with advanced fluids and chamber stability. The expected advantages of the MicroFlow® system are expanded chamber depth, improved cutting ability for a variety of lens densities and reduced fluid volume resulting in less endothelial cell loss. The MicroFlow® system was considered the appropriate control group to compare with AquaLase®.
The purpose of our study was to compare any changes in surgically induced astigmatism (SIA), corneal endothelial cell damage, postoperative recovery of visual acuity and change of corneal thickness after cataract surgery performed by using the AquaLase® and phacoemulsification in the MicroFlow® system.
Materials and Methods
In this prospective study, 19 patients (19 eyes) with cataracts (Lens Opacities Classification System (LOCS), version III3 nuclear grade below two) were included. The cataract was graded as the 4 grading scales of LOCS III using a biomicroscope. Therefore LOCS III nuclear grade below 2 means that the cataract had less than moderate nucleosclerosis. Nineteen eyes underwent cataract operation using AquaLase® and the control group (19 eyes) was selected by matching age, sex, systemic disease, grade of nuclear color and opacification, axis and magnitude of corneal astigmatism and corneal endothelial cell density. The opposite eye of each of the test patients was not included in the control group. One surgeon (C.K.J.) performed all surgical procedures in October of 2005. Preoperative examinations included visual acuity, intraocular pressure, slit lamp examination, fundus examination, A & B-scan, pachymetry, keratometry and specular microscopy.
Keratometry was measured using a manual keratometer (Topcon DM-4, Japan) and corneal thickness was measured using ultrasound pachymeter (Humphrey Instrument Inc.) preoperatively and at 1 week and 2 months postoperatively. Decimal visual acuity was measured preoperatively and at 1 day, 1 week, and 2 months postoperatively. Corneal endothelial cell density was measured using a specular microscope (Noncon Robo-CA SP-8000, JAPAN) preoperatively and at 2 months postoperatively. In addition, the coefficient of variation of cell size, and percentage of hexagonal cells were assessed. All endothelial parameters were measured in the central cornea. Surgically induced astigmatism was analyzed by the Law of Cosines and Sines4 method at 1 week and 2 months postoperatively.
After topical anesthesia, a self-sealing 3.2 mm temporal clear corneal incison was made by a diamond knife. After hydrodissection and hydrodelineation, the lens nucleus was divided by an Akahoshi prechopper. The lens was liquified using the AquaLase® of the Infinity Vision System (Alcon Laboratories, Fort Worth, Texas). In the control group the lens was phacoemulsified using a bevel-down phaco tip with the Millenium® (Stortz, U.S.A.) unit. Surgical settings of the AquaLase® and Millenium® unit were presented in Table 1. All patients had implantation of a foldable acrylic IOL. No corneal suturing was done.
SPSS software was used for statistical analysis. An unpaired t-test was used to test for interindividual differences and Pearson's bivariate correlation was used to examine the correlations between all of the numerical variables. A p value less than 0.05 was considered statistically significant and all p values reported were 2-sided.
Results
The mean age was 58.9±10.6 years in the AquaLase® group and 61.6±10.3 years in the MicroFlow® system group (p>0.05). The mean LOCS III classification nuclear grade was 1.16±0.37 in AquaLase® group and 1.16±0.37 in the control group (p>0.05). The mean preoperative magnitude of corneal astigmatism was 0.43±0.53 diopters in AquaLase® group and 0.63±0.52 diopters in the control group (p>0.05). The mean preoperative endothelial cell density was 2510±378/mm2 in AquaLase® group and 2616±303/mm2 in the control group (p>0.05). None of these preoperative factors were statistically significant between the two groups. Table 2 summarizes the preoperative patient data.
The mean SIA at 1 week postoperatively was 0.54±0.34 diopters in the AquaLase® group and 0.53±0.37 diopters in the control group (p=0.910). The mean SIA at 2 months postoperatively was 0.33±0.20 diopters in the AquaLase® group and 0.53±0.28 diopters in the control group (p=0.022, Fig. 1). The mean corneal endothelial cell loss at 2 months postoperatively was 4.5±24 % in the AquaLase® group and 10.2±10.8 % in the control group (p=0.417, Fig. 2A). The mean coefficient of variation at 2 months postoperatively was 0.332±0.050 in the AquaLase® group and 0.339±0.045 in the control group (p=0.686, Fig. 2C). The mean percentage of hexagonal cells at 2 months postoperatively was 52.1±8.70 in the AquaLase® group and 54.6±10.0 in the control group (p=0.461, Fig. 2D).

Endothelial cell loss (A), endothelial cell density (B), coefficient of variation of cell area (C), and percentage of hexagonal cells (D) in the AquaLase® group and control group at 2 months postoperatively. No statistically significant differences were found between the two groups.
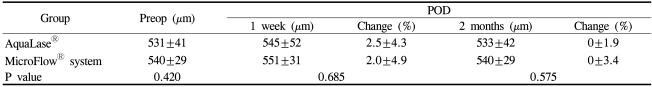
The uncorrected decimal visual acuity at 1 day, 1 week and 2 months postoperatively was 0.67±0.21, 0.86±0.22, 0.85±0.17 in the AquaLase® group and 0.65±0.30, 0.84±0.23, 0.87±0.13 in the control group (p>0.05, Fig. 3A). The distance-corrected decimal visual acuity at 1 day, 1 week and 2 months postoperatively was 0.82±0.28, 0.93±0.16, 0.95±0.09 in the AquaLase® group and 0.87±0.16, 0.95±0.10, 0.94±0.10 in the control group (p>0.05, Fig. 3B). The corneal thickness at 1 week and 2 months postoperatively was 545±52 µm, 533±42 µm in the AquaLase® group and 551±31 µm, 540±29 µm in the control group (p>0.05, Table 3).

Uncorrected visual acuity (VA) (A) and distance-corrected visual acuity (b) between AquaLase® group and control group after operation. There were no statistically significant differences between the two groups (p>0.05).
A statistically significant difference was found between the two groups only in the SIA at 2 months postoperatively (p=0.022). However, there were no correlations between any of the numerical variables (p>0.05).
Discussion
The AquaLase® handpiece warms a balanced salt solution up to 57℃ and the warmed saline is mixed with a peripheral irrigation fluid, causing the temperature of the mixed fluids to stabilize at 32℃ (R. Dimalanta, ASCRS Symposium on Cataract, IOL and Refractive Surgery, San Francisco, CA, April 12-16, 2003). Therefore, during the procedure, peripheral tissues are not damaged from the effect of temperature extremes. Furthermore, experimental measurement of internal wound temperature has shown that no incision heat is generated even at full power.1 So the advantage of the AquaLase® include lower corneal endothelial cell damage and no incisional burn compared with preexisting ultrasound phacoemulsification.2 However, there has not been objective research examining theses advantages except for the research about corneal endothelial cell damage after lens extraction using the fluid-based system in human cadaver eyes.5
The MicroFlow® system developed by Bausch & Lomb Surgical has high performance with advanced fluids and chamber stability and the proposed advantage that reduced fluid volume results in less endothelial cell loss. The MicroFlow® system was used as the operative instrument for the control group in the comparison with AquaLase®
We predicted that the fluid-base system in AquaLase® would induce less endothelial cell damage and wound burn compared with phacoemulficiation in the MicroFlow® system because it produces negligible heat. But the results show that only development of SIA was significantly different between the two groups (p=0.022) and there were no significant differences in the endothelial cell density, recovery of visual acuity or the change of corneal thickness.
The mean corneal endothelial cell loss at 2 months postoperatively was 4.5±24% in the AquaLase® group and 10.2±10% in the control group and there was no significant difference between two groups (p>0.05, Fig. 2A). This result is supported by similar results by Sandoval et al.5 They reported that the mean total damaged endothelial cells was 60.2±24.1/mm2 in the AquaLase® group and 60.4±42.6/mm2 in the ultrasound phacoemulsification group and there were no significant differences between the two groups in Human cadaver eyes (p>0.05). However, there was a high level of variability in cell loss for the AquaLase® group, which was not explained clearly, so it is necessary to further evaluate this result.
The surgical factors related with postoperative corneal edema are trauma, inflammation and surgical skills. The power and overall time required for phacoemulsification are also associated with corneal edema. Lundberg et al.6 have reported that the increase in pachymetry at 1 day postoperatively is strongly associated with a clinically significant corneal endothelial cell loss. Considering that recovery of visual acuity at 1 day postoperatively in the AquaLase® group was not significantly different from that in the control group (p=0.39, Fig. 3), we assume that corneal edema at 1 day postoperatively is likely to be similar in the two groups. At 1 week and 2 months postoperatively, corneal thickness was increased by 2.6±4.3%, 0±1.9% in the AquaLase® group and by 2.0±4.9%, 0±3.4% in the control group. There were no statistically significant differences between the two groups at 1 week and 2 months postoperatively (p>0.05, Table 3).
These results may indicate that remarkable improvements with phacoemulsification in the MicroFlow® system, surgical skill and ophthalmic viscosurgical devices has enabled us to decrease many complications, including corneal endothelial cell damage in cataract surgery. Also, patients with LOCS III nuclear grade more than 3 were not included in this study. Hayashi et al.7 reported that firmness of the nucleus was the most significant risk factor for endothelial injury because of mechanical contact with the nuclear fragments. And Walkow et al.8 reported that phacoemulsification time was a significant factor for central endothelial cell loss and it was highly correlated with the relative intensity of the phacoemulsification. In the case of a relatively soft nuclear cataract, it needs less time and less power to phacoemulsify the nucleus so that minimal mechanical trauma occurs. So there is no sufficient reason to develop statistically significant corneal endothelial cell damage. Bourne et al.9 have reported that no significant difference in overall corneal endothelial cell loss was found between modern phacoemulsification and extracapsular cataract surgery which did not produce heat. In their report, significant decreases in the corneal endothelial cell count were found only in the case of severe nucleosclerosis removed by phacoemulsification. Although AquaLase® has the advantage of less heat production compared to phacoemulsification in the MicroFlow® system, AquaLase® has shown limited ability in producing significant differences in corneal endothelial cell damage compared with the control group for extracting mild to moderate cataract.
The mean SIA at 2 months postoperatively was 0.33±0.20 diopters in the AquaLase® group and 0.53±0.28 diopters in the control group resulting in a significant difference (p=0.022, Fig. 1). This result was compared with other reports that SIA in conventional phacoemulsification was 0.71~1.20 diopters.10-12 The factors inducing corneal astigmatism after cataract operation are surgeon related factors including incision and suture method and the wound healing process. Bilinska et al.10 have reported that surgically induced astigmatism can be minimized with incisions in a clear cornea without suture. Huang FC and coauthors13 report that the differences between the SIA at 1 week and 1 month were significant, but the differences between 1 and 3 months and 3 and 6 months were not. This indicates that corneal topographic stability is reached between 1 and 3 months postoperatively. SIA by thermal injury also decreases spontaneously over several months.14 We suppose that the effect of thermal injury may be masked by other factors affecting SIA in the early postoperative period and could be detected after topographically stabilizing. Thermal damage that degenerates corneal collagen in the incision site occurs at 60℃.15,16 Because the same 3.2 mm clear corneal incision at the same temporal clear corneal site was done by one surgeon, SIA was likely to be induced mainly by heat greater than 60℃. The loss of adequate flow of irrigation fluid around the phacoemulsification tip and the use of excessive ultrasound power are the key factors in the development of phacoemulsification induced thermal injury.14,17 We suppose that this thermal injury can cause tissue shrinkage around the wound, leading to difficult wound closure, and wound leakage. But in AquaLase® the temperature of the water-jet pulse mixed with irrigation fluids stabilizes at 32℃ and the experimental measurements of internal wound temperature have shown that no incision heat is generated even at full power.1 Therefore, AquaLase® induces less surgically induced astigmatism than phacoemulsification in the MicroFlow® system although the phacoemulsification tip with the MicroFlow® system has an irrigation sleeve.
As mentioned above, there was a significant difference only in SIA at 2 months postoperatively between the AquaLase® group and the control group if AquaLase® was used to liquefy a relatively soft nucleus (LOCS III nuclear grade below 2). Consequently, in mild to moderate cataracts AquaLase® induces a better result than the MicroFlow® system only in SIA.
Recently Lehmann has reported that a hard nucleus (LOCS III nuclear grade over 3) is more easily removed by using the pre-chop method (Lehmann RP, AquaLase versus Phaco, Highlights of Ophthalmology 2005). Improvements in surgical skills including the pre-chop method, using a high vacuum, and the improvement of AquaLase® technology will enable us to use AquaLase® more easily in hard cataract surgeries.
In the future, AquaLase® will give us more advantages during cataract surgery as well as less SIA.