Peripheral Defocus and Myopia Management: A Mini-Review
Article information
Abstract
Myopia is the most common refractive error in the world, and its’ prevalence continually increases. The potential pathological and visual complications of progressive myopia have inspired researchers to study the sources of myopia, axial elongation, and explore modalities to arrest progression. Considerable attention has been given over the past few years to the myopia risk factor known as hyperopic peripheral blur, the focus of this review. The primary theories currently believed to be the cause of myopia, the parameters considered to contribute and influence the effect of peripheral blur, such as the surface retinal area or depth of blur will be discussed. The currently available optical devices designed to provide peripheral myopic defocus will be discussed, including bifocal and progressive addition ophthalmic lenses, peripheral defocus single vision ophthalmic lenses, orthokeratology lenses, and bifocal or multifocal center distance soft lenses, as well as their effectivity as mentioned in the literature to date.
Introduction
Contributing factors to myopia progression
Myopia is the most common refractive error worldwide, continuously advancing and spanning the entire literate world [1–3]. Untreated, progressive myopia can lead to complications affecting vision, ocular alignment, and physiological blindness [4–7]. Genetic and environmental factors influence myopia occurrence and progression, and some seem to be closely linked to each other [8–12].
Genome and candidate gene-based studies have identified over 600 loci connected to refraction and myopia. Still under investigation, these genes’ specific roles and clinical manifestations are not yet completely understood [13], as they often have multiple functions. Genes have been identified to be involved in synaptic transmission, calcium ion binding, cation channel activity, cell-cell adhesions, as well as plasma membrane function [14].
Light-dependent genes may affect the cell cycle and growth pathways. Therefore, lack of outdoor activity and high levels of education, which sometimes may be contiguous, are probably also important contributors [13,14]. Animal models suggest that exposure to sunlight stimulates retinal dopaminergic pathways, which then interfere with eye growth signaling pathways, thereby preventing excessive axial elongation [13]. Excessive and prolonged accommodation is also a possible catalyst for axial elongation by releasing chemical mediators which leads to cellular and biochemical changes in the retina, signaling changes to the choroid, and ultimately scleral and refractive error growth [15]. Hyperopic peripheral blur is an acknowledged risk factor for myopia development, while myopic peripheral blur in the retina may arrest this progression (Fig. 1) [16,17]. In recent years the search for a treatment that will halt myopia progression has yielded several options. Low-concentrated atropine, orthokeratology, bifocal or progressive addition ophthalmic lenses, and soft multifocal center-distance- peripheral-blur contact lenses have all shown effectivity [12,13,18].
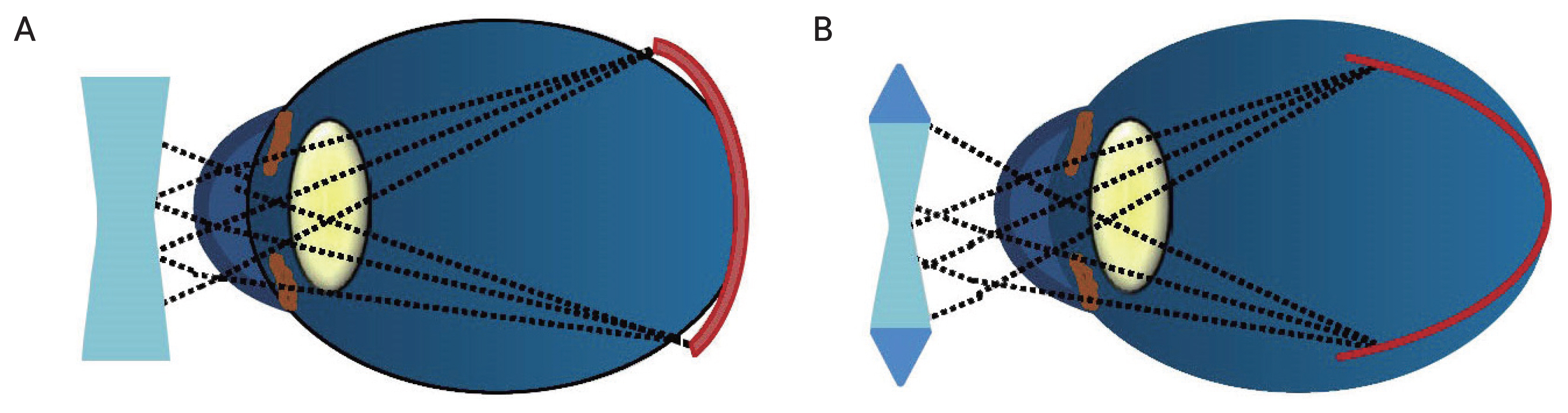
Myopic correction with single vision contact lenses or single vision spectacles correct myopia at both the fovea and the peripheral retina in equal amounts. This causes the myopic eye’s fovea and peripheral retina to be in different myopic states. (A) The peripheral retina is more hyperopic; therefore, equal myopic correction peripherally and centrally is likely to enhance myopia progression. (B) As illustrated, myopic correction with peripheral myopic defocus contact lenses or spectacle lenses correct the full degree of myopia at the fovea but create myopic defocus in the peripheral retina by providing additional positive power in the periphery, thus retarding myopia progression.
This article will discuss peripheral defocus as it pertains to myopia progression and the optical methods devised to date in an attempt to control it.
Search strategy
A thorough search of the professional literature in Medline, Scopus, and Thomson Reuters Web of Science databases and search engines was conducted. Search terms such as “Peripheral Defocus,” or “Peripheral Hyperopic Defocus,” or “Relative Peripheral Refraction,” and the words “Myopia,” “Myopia Progression,” “Myopia Management,” and “Myopia Control” were entered. The search yielded 137 peer-reviewed articles, and from the references of those articles, another 17 articles related directly to relative peripheral refraction and myopia progression were identified.
Theories of Relative Peripheral Defocus and of Myopia Progression
The influence of peripheral defocus on eye growth manifested in animal research has encouraged the exploration of optical methods for humans to control myopia progression. Smith et al. [19] ablated 13 rhesus monkeys’ foveae using photocoagulation to demonstrate the peripheral retina’s role in emmetropization. The refractive state was subsequently monitored using retinoscopy, keratometry, and A-scan ultrasonography. Refractive changes were noted even following foveal ablation [19].
Several studies exhibited the importance of the peripheral retina as the controlling area of defocus that can modify the growth and refractive state of the eye. Specifically, producing peripheral hyperopic defocus can cause axial myopia, whereas peripheral myopic defocus can even lead to axial hyperopia [13,20,21]. Researchers have found the baseline peripheral refraction in isolation does not predict the onset or progression of myopia [13,20,22]. The development of myopia associated with the adjustment from relative peripheral myopia to relative peripheral hyperopia was apparent in a study by Sng et al. [23] on children in Singapore.
There are two primary theories regarding the physiological progression of myopia. One theory hypothesizes that hyperopic retinal blur caused by a high lag of accommodation during near work accelerates axial elongation [24–26], and a second theory proposes that mechanical tension created by the ciliary body or crystalline lens restricts equatorial ocular expansion, thereby causing accelerated axial elongation [27].
Animal model studies suggest that form deprivation and retinal blur initiate a signaling cascade that leads to cellular and biochemical changes in the retina and the retinal pigment epithelium [28–31]. These chemical signals are transmitted through the choroid, causing changes in scleral extracellular matrix synthesis, which alters the biomechanical properties of the sclera, leading to ocular growth and a more myopic refraction [32,33]. Animal studies and models have further shown that the choroid plays an active role in emmetropization through thickness modulation to adjust the retina to the focal plane of the eye (choroidal accommodation) and through the release of growth factors that have regulatory scleral extracellular matrix remodeling potential [32,33]. Experimental studies have identified that several biochemical compounds, such as retinal dopamine, retinoic acid, and nitric oxide, are involved in axial length (AL) modulation [34–36].
Possible mechanisms driving axial growth include high levels of retinal blur caused by axial aberrations, form deprivation resulting from poor retinal image quality in distance vision, enhanced accommodative lags over time encouraging compensatory eye growth, and an absence of adequate cues to guide emmetropization [37,38]. While foveal defocus has been discussed as a promoter of myopia progression, which is part of the incentive to prescribed lenses with an addition for near work, the peripheral blur threshold seems to be a more significant influence.
A concept of local control has been raised, namely manipulating the visual environment in one area of the visual field, thereby influencing only the refractive state in the corresponding retinal area. This concept could raise the argument against peripheral defocus affecting refractive error at another location, such as the fovea, the standard location where myopia as well as AL are measured (at the retinal pole) and determined as the communicated measure for progression or lack thereof [39]. Typically, myopic eyes have relative peripheral hyperopic defocus in the horizontal meridian, indicating it may be a potential growth signal; most research measurements are taken only at the horizontal, infrequently at the vertical meridian. One study measured the peripheral refractive error in five peripheral meridians (up to 40° from the fovea). It concluded that asymmetric profiles seemed to be influenced less by defocus multifocal contact lenses than those with symmetric profiles, especially when skewed temporally or nasally [40–42]. This concurs with a study that presented that patients with asymmetric peripheral refraction were less prone to myopia progression, suggesting they may also be less receptive to changes that induce myopia, such as peripheral defocus [40,41].
A study by Mutti et al. [20] noted that in the 2 to 4 years prior to myopia manifestation there was an increased rate of change towards a negative refractive error, increased AL, and a more hyperopic relative peripheral refractive error (measured at 30º from the fovea). These presentations may assist in predicting the onset of myopia [20].
Peripheral refraction: location and degree
Sensitivity to blur decreases with increased distance from the fovea, which some feel implies that greater magnitudes of defocus are required in the periphery to result in detectable blur. Others contend that just as with accommodation, capable of being stimulated at much lower levels than the depth of focus, the amounts of peripheral defocus may not have to exceed the depth of focus to trigger myopia progression. Additionally, blur sensitivity usually describes the ability to perceive the focus of the image, which requires neural processing. Still inconclusive is the optimal level of blur required to influence eye growth. The feedback loop for emmetropization seems to occur at the retina level, perhaps due to a specific type of ganglion cells [40,41].
While it is also unclear what level of defocus and at what retinal eccentricity might influence foveal refraction, most studies successfully influence eye growth when targeting the area between 20° to 40° from the visual axis. The percentage of the surface area required to affect myopia progression has not yet been determined [40,41].
It is important to appreciate that regardless of the lens type, one that corrects a higher amount of central myopia will result in a more hyperopic peripheral shift than a lower powered lens. Therefore, a higher degree of myopia potentially influences continuation of growth more than a lower degree [22]. As mentioned, undetermined is the degree of blur required to influence this hyperopia or the refractive status of the periphery (emmetropic, myopic, and to what degree); therefore, the amount of addition required in the periphery is also under consideration. So still unestablished is the necessary depth of defocus, how much retinal area of blur is needed to be effective, and whether an image can be too blurry to be effective.
Peripheral defocus optical devices and their effectivity
Single vision (SV) ophthalmic lenses used to correct myopia have been shown to increase hyperopic defocus at the retina’s periphery. As the amount of myopia correction increases, so does the peripheral hyperopic defocus magnitude [43].
Optical Devices that Provide Peripheral Defocus and Their Effectivity
Spectacle lenses
1) Bifocal ophthalmic lenses
One of the earliest optical modalities used in an attempt to manage myopia progression is the bifocal lens. In addition to addressing accommodative lag and binocular imbalances, an executive bifocal lens worn over 3 years resulted in a 39% slowing of myopia progression whereas a standard “D” segment bifocal spectacle lens with base-in prism had a 50% treatment effect [43]. Walline [44] conducted a myopia control review where they discovered that both bifocal and multifocal ophthalmic lenses have some effect on controlling myopia but were not sufficient, in his view, to recommend using this modality, even for those with a high accommodative lag and near-point esophoria. The increased positive power for near may potentially have a negative effect on the oculomotor balance in children with orthophoria, reducing myopia progression’s effectiveness [45,46]. A trial using a base-in prismatic bifocal lens showed a more significant effect amongst children with low lags of accommodation due to reduced convergence and lens-induced exophoria [44,47]. In the case of the children with a low lag of accommodation, the change in AL over the 3 years for those wearing the prismatic bifocal was 0.46 mm. In contrast, the change in AL in those wearing the standard bifocal was 0.6 mm over the same time period [44].
2) Progressive ophthalmic lenses
A study comparing myopia progression between children wearing SV spectacles and progressive addition lenses (PALs) reported that those with superior myopic defocus (PALs) had significantly less central myopia progression than those with superior hyperopic defocus (SV spectacles) [48]. Studies that compared the use of PALs with SV lenses showed a statistically significant but clinically insignificant change in myopia progression between them of 0.20 diopters (D) over a 3-year period [24,26,48,49]. It should be mentioned that some studies have observed that the children wearing PALs sometimes fail to use the near reading zone for near viewing [50]. The indication of sectorial defocus influencing sectorial AL inspired researchers to develop optical solutions that would provide equal defocus over the entire circumference.
3) Peripheral defocus ophthalmic lenses
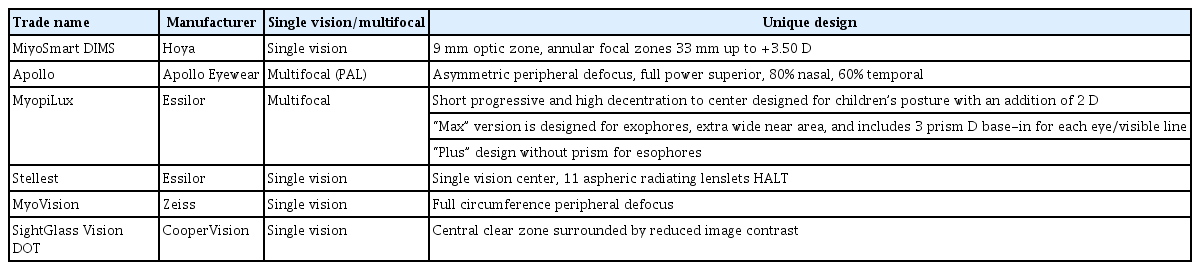
An ophthalmic lens with peripheral defocus is limited, as its’ effect is potentially altered in primary versus other gaze directions. These designs are the newest module in the myopia management arena to be developed and studied. (Table 1).
An SV lens called the defocus incorporated multiple segments (DIMS) [51], developed at Hong Polytechnic University and manufactured by Hoya, comprises a central optical zone of 9 mm in diameter which corrects the full distance refractive error and has annular multiple focal zones with multiple segments up to 33 mm in diameter with a relative positive power of +3.50 D [40,52]. The diameter of each of these segments is 1.03 mm. Children in one study wearing these lenses for 2 years had an increase in myopia of − 0.3 D, whereas children wearing SV lenses had an increase of myopia of −0.93 D. The increase in AL was 0.21 and 0.53 mm, respectively. The strongest effect was noted during the first 6 months of lens wear, possibly due to the higher myopia progression in the SV lens group during this time [40,41].
A PAL named the Apollo lens (Apollo Eyewear) comprises an asymmetrical myopic defocus design with a three-stage myopic defocus zone. These include a +2.50 D full positive power superior zone, an 80% full myopic defocus power nasal zone, and a 60% full myopic defocus power temporal zone [40,52].
Perifocal ophthalmic lenses were assigned to children of 7 to 14 years with progressive myopia from −1.00 to −6.00 D in a study. These lenses allow differentiating correction of the central and peripheral refraction of the eye along the horizontal meridian, thereby correcting or reducing peripheral hyperopia. In the 15° zone, 100% of the eyes formed myopic defocus, which averaged −0.05 ± 0.1 D in temporal 15°, −0.25 ± 0.16 D in nasal 15°, and −0.44 ± 0.03 D in temporal 30°. In the nasal 30° zone, the hyperopic defocus decreased by four times and amounted to 0.38 ± 0.03 D. The rate of myopia progression decreased from 0.80 D of baseline values to 0.17 D at 4 to 5 years of follow-up [53].
Essilor’s contribution to the myopia control ophthalmic lens modality is the Myopilux series which includes two designs: (1) an executive bifocal with an add power of +1.50 D and design; (2) the same design with 3 prism D base-in incorporated in the near segment of each lens resulting in a total of 6 prism D base-in in the near segment, named Myopilux Max. This lens is based on research done by Cheng et al. [47].
Recently Essilor also introduced the Stellest lens, which incorporates a central SV center and 11 aspheric lenslets radiating outward. These 11 concentric rings have the distance correction between them and are centered around a 9 mm-diameter clear zone [54]. The power rings create signals in front of the retina which slows eye elongation, a design named Highly Aspherical Lenslet Target (HALT). These lenses do not focus light on two distinct surfaces, but deviate rays of light continuously, creating a three-dimensional quantity of light in front of the retina which they call a volume of myopic defocus. The results of a 1-year study including 170 myopic children were as follows: in the HALT group, myopia progressed by −0.27 D compared to −0.48 D in those wearing SV spectacle lenses (SVL), and the AL change over the same period was 0.13 mm in the HALT group versus 0.36 mm on the SVL group [55]. Both the MiyoSmart (Hoya), slightly aspherical lenses, and the Stellest lens, highly aspherical lenslets, are based on the peripheral retinal defocus theory and both have an effectivity of about 60% [55,56].
Shamir Optical Industry is currently investigating in clinical trials a spectacle lens named MyLens for myopia management. The study compares the rate of myopia progression in children wearing this lens to children wearing a standard spherical or toric single focus ophthalmic lens. The design comprises a unique asphericity. Peripheral refraction and ocular aberration are evaluated. In this study, 61 participants were randomly recruited. The title of the study, which began in January 2016, is called Shamir Aspheric Ophthalmic Lenses (MyLens) for Myopia Control (ClinicalTrials.gov identifier: NCT05477329) [57].
A second study is being conducted in Israel. This is a controlled, double-masked interventional trial where 136 participants have been randomly recruited. The goal is to evaluate the effect of the MyLens compared to a SV lens worn by children in Israel [57]. The test lens is manufactured in a standard freeform design like a standard PAL. The study began in June 2021 and is intended to be a 3-year study [57].
Contact lens
1) Spherical single vision soft contact lenses versus single vision spectacles
A comparison between peripheral refraction in ophthalmic lenses and standard spherical soft contact lenses (SCLs) has been investigated. While one study found a myopic defocus (when prescribing for full distance refractive error) during SCLs wear [58], most found hyperopic defocus with both correction modalities, whether undercorrected, on-refraction, or overcorrected [59]. Walline et al. [60] examined 247 SCL wearers and 237 spectacle wearers aged 8 to 11 years and found that soft contact lenses did not significantly affect corneal curvature or axial elongation compared to spectacle wearers.
2) Orthokeratology
Corneal reshaping lenses are worn at night while sleeping. They flatten the central cornea while steepening the mid-peripheral cornea, primarily involving the epithelial layer. This reduces the relative peripheral hyperopia, which in turn seems to slow axial elongation. Children that had been wearing orthokeratology lenses over 2 years demonstrated an increase in AL and vitreous chamber depth of an average of 0.14 mm/yr compared to an average growth of 0.27 mm in children that did not wear these lenses. Most studies in this area have shown the overall decrease in myopia progression to be around 50% [10,61,62], a significant impact that can be linked to the peripheral defocus effect [61–63]. Multiple companies around the globe are producing these lenses with more customized options to treat astigmatism and to better control the treatment zone and peripheral defocus area.
3) Bifocal and multifocal soft contact lenses
Short-term studies on animals and children have presented that soft bifocal contact lens designs can slow myopia progression. To date, peripheral defocus contact lenses have been shown to be superior to peripheral defocus ophthalmic lenses, but studies are being conducted on newer technologies that may reveal new data [36,64]. The combined potential benefit of peripheral defocus contact lenses and their bifocal effect on accommodation has been reported, and investigations continue to substantiate this [33,65–67].
Both concentric ring bifocals and peripheral addition multifocal SCLs have been shown to be clinically effective for decreasing myopia progression in school-aged children, with an overall myopia control rate of 30% to 50% over 2 years. Concentric ring bifocal SCLs seem to have a more significant effect than peripheral addition multifocal SCLs [59]. The Bifocal Lenses in Nearsighted Kids (BLINK) study showed that 51% of the participants progressed more than −1.00 D over a 3-year period compared to those wearing +1.50 D addition and +2.50 D addition multifocal contact lenses who progressed less than −0.85 D and −0.56 D, respectively [68].
Overall, the effect of bifocal SCLs on myopia control in the literature varies, ranging from 25% to over 70% reduction in myopia progression. Some of these studies report results after only 1 year. The more variable results have been observed with soft bifocal contact lenses in comparison to orthokeratology, which may be due, among other reasons, to differences in lens design, compliance with wearing the contact lenses, or the number of hours of wear [32].
4) Contact lenses designed to manage myopia
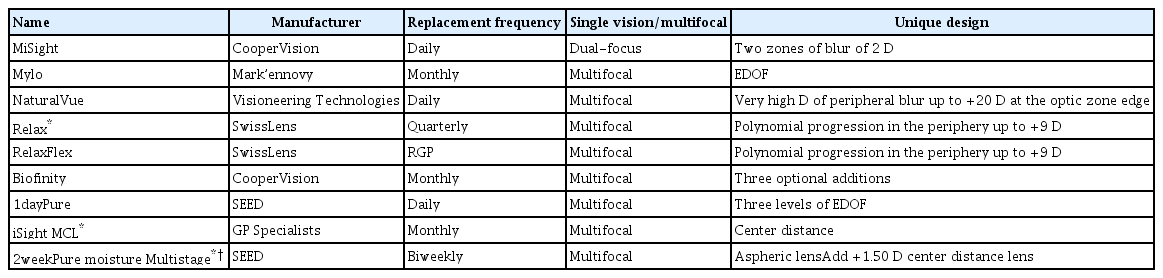
In general, increasing addition plus power will increase treatment efficacy and also spread over a larger area of the retina. However, this benefit can sometimes negatively impact vision, such as contrast sensitivity or acuity, and a balance between visual quality and treatment zone size and location engenders the multiple designs emerging over recent years (Table 2) [69–71].
5) Dual-focus design
Commercially known as MiSight (CooperVision), these lenses have a central zone with a diameter of 3.36 mm that corrects the refractive error and concentric treatment zones that create 2.00 D of simultaneous myopic retinal defocus during distance and near viewing. The defocus is comprised of two alternating treatments and two refraction correcting zones causing peripheral defocus on the retina, creating dual-focus viewing. The trial conducted by Anstice and Philips [72] showed that eyes wearing these contact lenses had significantly less axial elongation than eyes wearing SV lenses. Following this study, Chamberlain et al. [36] conducted a multicenter study in several countries over 3 years. The refractive error progression in the 1st year was 0.40 D less compared to the control group. In the 2nd year the progression was 0.54 D less, and in the 3rd year 0.73 D less than the control group. The axial elongation change was as follows: at 12 months, the AL change in the control group was 0.24 mm compared to 0.09 mm in the MiSight group; at 24 and 36 months, the AL change was 0.24 and 0.3 2mm less than the control group, respectively. In terms of refraction, the myopia control effect after 3 years was 59% and in terms of AL control, the effect was a 52% decrease [36].
6) Extended depth of focus contact lens
The extended depth of focus lenses used in a study is commercially named Mylo (Mark’ennovy). There are two options of extended depths of focus available, including higher-order aberrations equivalent to additions of +1.75 D (lens no. 3 in the study) and +2.50 D (lens no. 4). When examined after 2 years, the lenses had slowed myopia progression by 32% and 26%, respectively [35].
7) Multifocal with high peripheral blur
The NaturalVue multifocal 1-day contact lens (Visioneering Technologies) has a continuous gradual decrease in myopic power which begins very close to the center of the lens. It is designed to provide approximately 3.5 D of relative plus power at a radius of 2.6 mm and approximately an additional 4 D of relative plus power at 3 mm, continuing at a far more gradual pace till the end of the optic zone. This is the largest degree of peripheral plus power commercially available in a center-distance multifocal contact lens. This lens has exhibited high potential at halting myopia progression in a multicenter case series analysis of 32 patients. Approximately 98% showed a decrease in annual myopia progression, 91% showed a 70% decrease or more, and a few patients exhibited myopia regression [34].
8) Acuvue Abiliti 1-day lens
Johnson & Johnson developed a daily SCL for myopia management. The researchers examined two lens prototypes: enhance efficacy (EE) design and enhance vision (EV) design [73]. The EE was designed to increase myopia control efficacy by introducing a greater amount of plus power than the standard multifocal or dual-focus designs, while still allowing good visual performance [73]. Both lenses were made up of two concentric, annular zones with +7.00 D coaxial plus power to treat myopia [73]. These annuler treatment zones are positioned closer to the lens center in the EE design than the EV design, and the EE design also has an additional +10 D coaxial treatment zone to assist in myopia control without adversely impacting vision [73]. After 6 months of 8 hours of daily wear, the EE lens presented superior results regarding clear visual acuity and myopia control efficacy [73]. The EE lens reduced axial elongation by 0.105 mm compared to the SV lens used in the study after 6 months of wearing the lens [73].
Future Studies
Future studies must clarify several unanswered questions, for example, which visual regions other than the fovea are most impactful in controlling myopia. Researchers are exploring the impact of influencing AL growth monocularly in cases of anisometropia [74].
A study comparing the efficacy between the DIMS SV lens and the Apollo PAL began in October 2019 and is currently underway [52]. SightGlass Vision is a clinical-stage research and development company conducting a three-arm trial comparing a novel SV ophthalmic lens design to SV spectacles [75,76]. The Personalized Addition Lenses Clinical Trial (PACT) study is comparing a customized PAL to standard +2.00 D addition PALs and SV lenses [77].
A recent study was published which compared MiSight to two uniquely designed myopia control soft lenses under development. While MiSight is a symmetric design, meaning the prescription and defocus is consistent independent of lens rotation, these lenses are using what the manufacturers (Contaflex 42, Brighten Optix Corp.) call Spatio Temporal Optical Phase (S.T.O.P) technology, whereby the optic zone has rotationally asymmetric power maps which are designed to have varying power distributions along different meridians. The asymmetric design and on-eye rotation provides an optical cue that is dynamic, that is, the areas of induced blur change as the lens rotates on the eye. This dynamic optical presentation showed comparable visual performance, binocular and accommodative function to MiSight, but still has not been compared regarding myopia progression inhibition capabilities [78].
Mutti et al. [20] concluded that the relative slower rate of change after myopia onset in myopia progression, axial elongation, and peripheral hyperopia suggests multiple factors may influence myopia progression. Studies have begun to explore the potential of combination therapies for myopia control, where perhaps each peripheral defocus modality has minimal to moderate benefit, but the combination with another effective alternative modality, such as low-dose atropine, may enhance effectivity [79–81].
While incomplete and still under investigation, the published data to date suggests that after orthokeratology, the most effective soft lens for myopia control is MiSight, manufactured by CooperVision and the two best spectacle lenses are MiyoSmart, manufactured by Hoya, and Stellest, manufactured by Essilor (Table 3 and Fig. 2) [34–36,47,52,54,55,75,76,82–85].
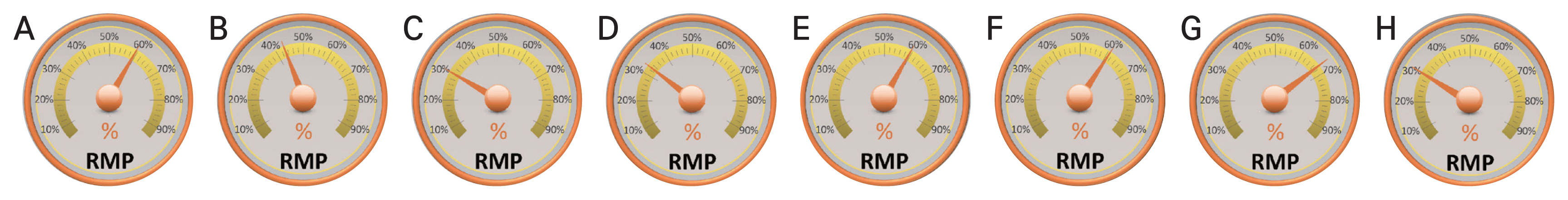
Reduction of myopia progression (RMP) expressed as the percentage of sphere equivalent decrease. A 3-year study: (A) dual-focus MiSight 1 day (CooperVision) and (B) multifocal Biofinity (CooperVision) contact lenses. A 2-year study: extended depth of focus (C) Mylo (Mark’ennovy) and (D) 1dayPure (SEED) contact lenses. A 3-year study: (E) single vision MiyoSmart (Hoya), (F) multifocal Myopilux (Essilor), (G) single vision Stellest (Essilor), and (H) single vision MyoVision (Zeiss) spectacle lenses.
Conclusion
In summary, myopia progression is a complex condition where multiple factors influence each other and treatment effectiveness. This article focused on the peripheral blur signal on the retina. While peripheral myopic defocus has proven to be a potent trigger to decrease myopia progression, the amount of defocus necessary is unclear. One possibility is that there is a dose-response relationship, meaning more significant amounts of defocus result in greater reductions in myopia progression. Another possibility is that any amount of myopic peripheral defocus above some threshold acts as a signal to slow myopia progression and the location is the important factor. Alternatively, or in combination, perhaps more significant reductions in myopia progression are possible as more peripheral locations experience myopic defocus. Factors such as myopia onset’s age, myopia’s depth, ethnicity, and environmental factors further impact treatment effectivity and the understanding and extrapolation of research data.
Acknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: None.