Comparison of Clinical Outcomes among Conventional Scleral Fixation, Retropupillary Iris-claw Intraocular Lens Implantation, and Intrascleral Fixation
Article information
Abstract
Purpose
To compare the efficacy and safety of conventional scleral fixation (C-SF), retropupillary iris-claw intraocular lens (RP-IOL) implantation, and intrascleral fixation (ISF).
Methods
This retrospective observational study included 58 patients (58 eyes) who underwent C-SF (23 eyes), RP-IOL (23 eyes), and ISF (12 eyes) by a single surgeon at Samsung Medical Center from October 2017 to July 2020 and were followed up for at least 6 months. This study analyzed various clinical outcomes before surgery, and 1 day, 1 week, 1 month, 3 months, and 6 months after surgery.
Results
Six months after surgery, best-corrected visual acuity in logarithm of minimum angle of resolution was 0.08 ± 0.10, 0.08 ± 0.16, and 0.03 ± 0.04 in C-SF group, RP-IOL group, and ISF group, respectively, and there was a significant improvement in each group compared to preoperative best-corrected visual acuity. All groups showed a significant increase in astigmatism postoperatively, but no between-group differences were observed. The prediction error was −0.15 ± 0.77, 0.56 ± 0.62, and 0.44 ± 1.00 diopters in the three groups, respectively, indicating RP-IOL group and ISF group for hyperopic shift. The three groups did not differ in terms of absolute prediction error. Six months after surgery, the corneal endothelial cell counts were 2,073 ± 691, 2,014 ± 692, and 1,712 ± 891 cells/mm2, respectively, which were lower than before surgery. IOL dislocation occurred in five eyes only in RP-IOL group, two of which underwent two reoperations, and reenclavation was performed smoothly without complications in all cases.
Conclusions
Although the frequency of IOL dislocation in RP-IOL group was higher than that in the other groups, it can be reenclavated relatively easily. As a method of secondary IOL fixation, both RP-IOL implantation and ISF were as effective as conventional scleral fixation.
With increase in life expectancy and dramatic improvement in cataract surgery techniques, the number of patients undergoing cataract surgery has increased. Consequently, complications following cataract surgery are increasing, a representative complication being intraocular lens (IOL) dislocation. In a nationwide Korean cohort, the incidence of IOL dislocation was 908 per one million person-years [1], and the cumulative probability of IOL dislocation was 2.73% per person for 8 years after cataract surgery [2].
Various methods of IOL fixation, such as conventional scleral fixation (C-SF), retropupillary iris-claw IOL (RP-IOL) implantation, or sutureless intrascleral fixation (ISF), have been developed in patients without sufficient capsular support. Scleral fixation was first introduced by Malbran et al. [3] in 1986. C-SF has been used for decades and is familiar to many surgeons; however, the surgical procedure is complex and time consuming. In addition, tilting and decentration of the IOL and suture-related complications (e.g., suture exposure, infection, or suture loosening) may occur [4–6].
In 1971, Worst et al. [7] first introduced an iris-claw IOL made of polymethyl methacrylate that was fixed on the plane of the iris. This IOL did not interfere with the movement of the iris or the iridocorneal angle. Since then, Artisan (Ophtec BV, Groningen, Netherlands) and others have appeared in the market as a RP-IOL and have produced satisfactory results. RP-IOL implantation has several advantages: the surgical technique is easier and takes less time than C-SF technique [8,9]. However, there is also the possibility of iris damage, chronic inflammation, cystoid macular edema, and disenclavation [10–12].
ISF, a method of sutureless fixation of haptics to the sclera by creating a scleral tunnel, was first introduced by Gabor and Pavlidis [13]. Subsequently, several modified methods were introduced [14–16]. Because ISF does not require sutures, there are no suture-related complications. However, haptic tip deformation, breakage during externalization, and haptic slippage can occur [17,18].
To the best of our knowledge, few studies have compared the clinical outcomes between C-SF, RP-IOL implantation, and ISF. Moreover, consideration of the relatively long-term clinical results is lacking. Therefore, this study aimed to assess the clinical results over 6 months by analyzing the medical records of patients with aphakic eye or IOL dislocation who underwent C-SF, RP-IOL implantation, and ISF by a single operator.
Materials and Methods
Ethics statement
This study adhered to the tenets of the Declaration of Helsinki. The Institutional Review Board of the Samsung Medical Center approved the research protocol (No. 2021-11-033) and waived the need for informed consent.
Patient selection and outcome variables
In this study, 58 eyes of 58 patients who underwent secondary IOL fixation by a single surgeon at Samsung Medical Center for dislocated IOL or aphakic eye between October 2017 and July 2020 and were observed for more than 6 months, were retrospectively reviewed. Overall, 23 eyes of 23 patients with C-SF, 23 eyes of 23 patients with RP-IOL implantation, and 12 eyes of 12 patients with ISF were included in the study. Uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), intraocular pressure (IOP), corneal endothelial cell count (ECC), and manifest refraction of all patients in all groups were measured before surgery, and 1 day, 1 week, 1 month, 3 months, and 6 months after surgery. Postoperative complications were also recorded. Patients with corneal opacity, corneal dystrophy, prior corneal transplantation, macular degeneration, serious unrecovered retinal and choroidal disease affecting visual acuity, advanced glaucoma, or suspected amblyopia were excluded.
Prediction error (PE) was defined as the value obtained by subtracting the predicted spherical equivalent (SE) of the IOL formula from the postoperative SE at 6 months after surgery, and the absolute value of the PE was defined as the absolute PE. Positive and negative PEs indicated hyperopic and myopic shifts in refraction after surgery, respectively.
IOL power was calculated with the SRK-T formula using ARGOS (Carl Movu Inc., Santa Clara, CA, USA). In C-SF, the IOL is fixed anteriorly compared to when it is placed in the capsular bag, resulting in myopic shift. Although the degree of myopia differs depending on the length of the axial length, it is recommended to select an IOL power that is 0.5 diopters (D) to 1.0 D less than the power for in-the-bag fixation [19]. The IOL in ISF is expected to be positioned at a similar position with C-SF. In this study, an IOL power of 0.5 D less than the target diopter was selected in the conventional scleral fixation group and ISF group. For RP-IOL implantation, the IOL manufacturer recommended an A-constant for the SRK-T formula of 116.9 for retropupillary implantation, so the IOL power closest to the target diopter among the calculated values was chosen. Postoperative hypotony and increased IOP were defined as ≤6 mmHg and ≥21 mmHg, respectively. Optical coherence tomography (OCT; Spectralis, Heidelberg Engineering, Heidelberg, Germany) for macular status was performed preoperatively and 1 month after surgery in all patients. Cystoid macular edema (CME) was defined as a case in which the central macular thickness on OCT was ≥300 μm. Significant anterior chamber inflammation was defined as an anterior chamber reaction greater than or equal to 3+.
Surgical techniques
All surgeries were performed by a single experienced surgeon (DHL) under retrobulbar anesthesia using 2% lidocaine (lidocaine HCL Inj. 2%; Daihan Pharm Co., Seoul, Korea), and topical anesthesia using 0.5% proparacaine hydrochloride (Alcaine; Alcon, Puurs, Belgium). C-SF was performed in 23 eyes. A 3.5-mm corneal incision was made on the superior side and two conjunctival flaps were made around the 2 and 8 o’clock limbus to expose the sclera. A double-armed 10-0 polypropylene (Prolene; Ethicon Inc., Somerville, NJ, USA) was pierced through the sclera 2 mm from the limbus, and then withdrawn through the limbus on the opposite side. The needle was cut, and the thread was pulled out through the corneal incision and fixed at the outer third of the haptic of three-piece IOL (model YA60-BBR; Hoya, Tokyo, Japan). The procedure was performed symmetrically on the opposite side in the same manner. After inserting the IOL into the posterior chamber, the thread exposed over the sclera was properly pulled, and the tension was adjusted and knotted such that the IOL was centered without tilting. The corneal incision was sutured with 10-0 nylon (Ethilon, Ethicon Inc.), the exposed knot was covered with a conjunctival flap, and conjunctival suture was performed with 10-0 nylon. The corneal suture was removed 1 week after surgery.
RP-IOL implantation was performed in 23 eyes using the following procedure: a 6-mm corneal incision was made on the superior side and a RP-IOL made of poly-methyl methacrylate (Artisan aphakia IOL model 205, Ophtec BV) was inserted. After rotating to the horizontal position with the lens forceps, the lens was secured with the lens forceps such that the center was aligned with the pupil, and then the two haptics were gently pushed behind the iris. The iris was lifted slightly from the rear to the front to reveal the shape of the IOL support claw so that it could be recognized from the front of the iris. Iris tissue was inserted into the claw with a long microspatula, and the two claws were fixed to the iris. The corneal incision was sutured using 10-0 nylon (Ethilon) and stitch out was done 1 week after operation.
ISF was performed in 12 eyes using flanged intrascleral IOL fixation with the double-needle technique [16]. A 3.5-mm corneal incision was made and the three-piece IOL (Tecnis model ZA9003; Abbott Medical Optics, Santa Ana, CA, USA) was inserted into the anterior chamber. The trailing haptic was left outside to prevent the IOL from falling into the vitreous cavity. An angled sclerotomy was performed using a 30G needle (TSK Ultra Thin Wall Needle; TSK Laboratory, Tochigi, Japan) 2 mm away from the limbus, and the leading haptic was inserted into the lumen of the needle. On the 180° opposite side, another sclerotomy was performed, and the trailing haptic was inserted into the lumen of the needle using forceps in the same manner as the first. Each haptic was then pulled out onto the conjunctiva simultaneously using the double-needle technique. Both haptic ends were cauterized using an ophthalmic cautery device (Accu-Temp Cautery; Beaver Visitec, Waltham, MA, USA) to create a 0.3-mm sized flange. The haptic flange was pushed into the scleral tunnels. The corneal incision was sutured with 10-0 nylon (Ethilon) and stich out was performed 1 week after surgery.
Statistical analysis
Statistical analysis was performed using IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA). The Mann-Whitney U-test was performed to compare the two groups at one time point, the Kruskal-Wallis test was used to compare the three groups at one time point, and the Wilcoxon signed-rank test was used to compare before and after conditions within the group. A p-value of less than 0.05 was considered statistically significant. Categorical variables were expressed as numbers (%), and continuous variables were expressed as mean ± standard deviation.
Results
Patient characteristics
The results were compared and analyzed by dividing the patients into C-SF group, RP-IOL group, and ISF group. Table 1 shows the mean preoperative clinical data and the statistical significance of the differences between the groups for each parameter. There were no differences among the three groups in terms of sex, age, UCVA,
Visual outcomes
In all three groups, UCVA and BCVA improved significantly 6 months after surgery, and there was no significant difference according to the surgical method (Table 2). One week after the operation, UCVA in logarithm of minimum angle of resolution was 0.74 ± 0.79 in C-SF group, 0.66 ± 0.49 in RP-IOL group, and 0.65 ± 0.43 in ISF group, indicating a significant improvement compared to before the operation (Fig. 1). BCVA at 1 month after surgery was 0.14 ± 0.15 in C-SF group, 0.17 ± 0.23 in RP-IOL group, and 0.08 ± 0.05 in ISF group, and the change in mean preoperative BCVA was significant only in RP-IOL group. No significant differences were observed between the groups throughout the follow-up period (Fig. 2).

Mean UCVA, BCVA, astigmatism, ECC, macular thickness, and statistical significance of differences between the three groups before and after the operation
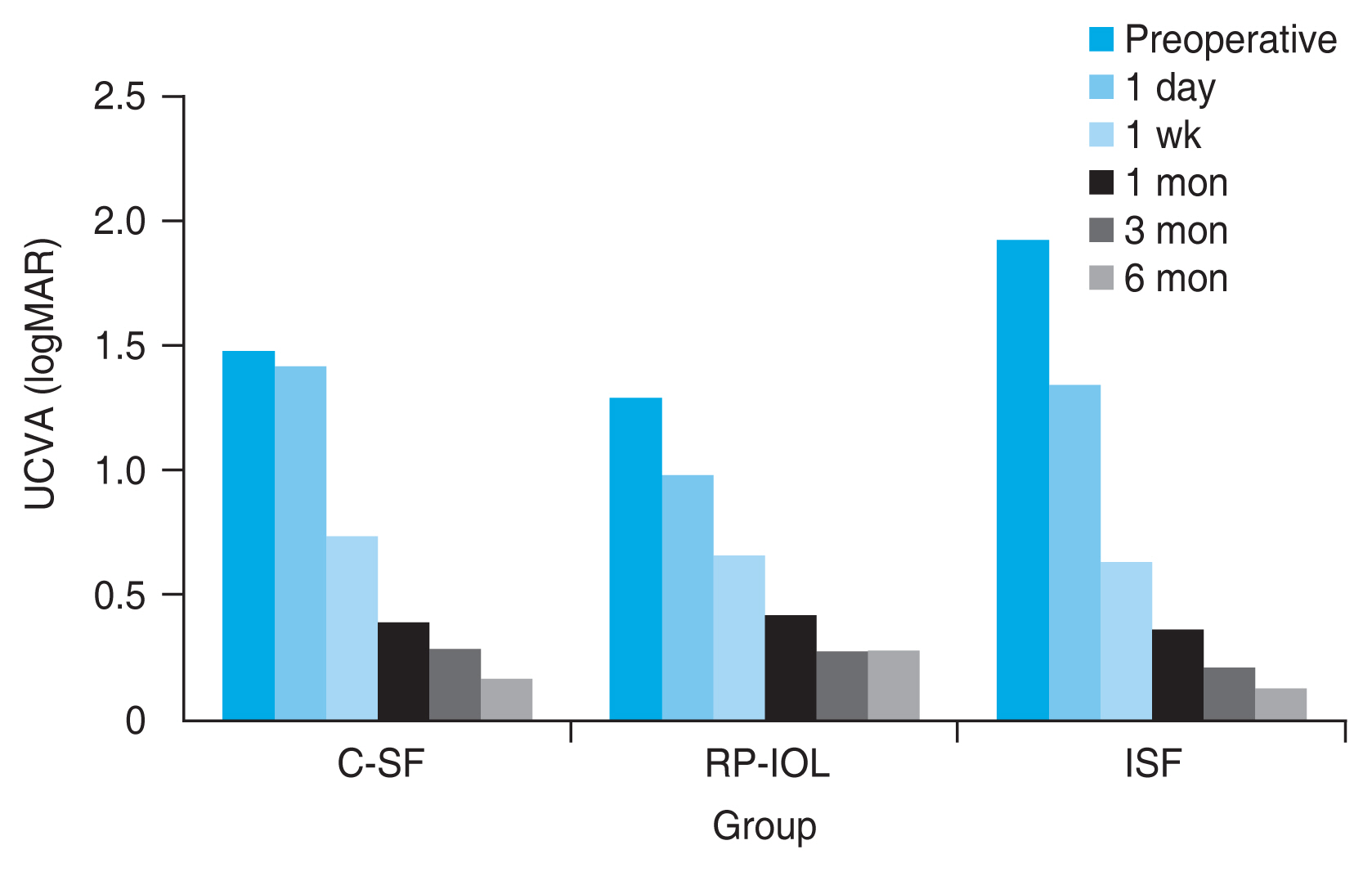
Changes in postoperative uncorrected visual acuity (UCVA) over time after conventional scleral fixation (C-SF), retropupillary iris-claw intraocular lens (RP-IOL) implantation, and intrascleral fixation (ISF). After 6 months of surgery, UCVA was significantly improved in all three groups. There were no differences between the three groups throughout the entire follow-up period (p > 0.05, Kruskal-Wallis test). logMAR = logarithm of the minimal angle of resolution.

Changes in postoperative best-corrected visual acuity (BCVA) over time after conventional scleral fixation (C-SF), retropupillary iris-claw intraocular lens (RP-IOL) implantation, and intrascleral fixation (ISF). After 6 months of surgery, BCVA was significantly improved in all three groups. There were no differences between the three groups throughout the entire follow-up period (p > 0.05, Kruskal-Wallis test). logMAR = logarithm of the minimal angle of resolution.
Refractive outcomes
Astigmatism measured by manifest refraction at 6 months after surgery was 1.47 ± 0.88 D in C-SF group, 0.97 ± 0.84 D in RP-IOL group, and 1.33 ± 0.94 D in ISF group. Astigmatism increased in all three groups compared to before surgery, but no difference was observed between the three groups (Table 2). PE was -0.15 ± 0.77, 0.56 ± 0.62, and 0.44 ± 1.00 D in the three groups, respectively, indicating RP-IOL group and ISF group for hyperopic shift. Absolute PE was 0.60 ± 0.49 D in C-SF group, 0.64 ± 0.53 D in RP-IOL group, and 0.90 ± 0.56 D in ISF group, and there were no between-group differences (Table 3).
Endothelial cell count
The ECC at 6 months after surgery was 2,073 ± 691 cells/mm2 in C-SF group, 2,014 ± 692 cells/mm2 in RP-IOL group, and 1,712 ± 891 cells/mm2 in ISF group, and no significant difference was observed between the three groups (Table 2). There was also no difference in ECC loss between the three groups, which is the difference between the preoperative and 6 months postoperatively. In all groups, ECC was significantly reduced postoperatively but remained above 1,700 cells/mm2 (Fig. 3).
Postoperative complications
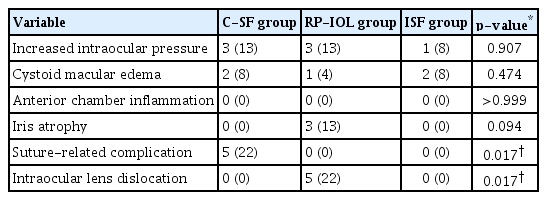
Increased IOP (IIOP) was observed in three eyes in C-SF group, three eyes in RP-IOL group, and one eye in ISF group (Table 4). In the IIOP cases, it ranged from 23 to 34 mmHg in C-SF group, 24 to 31 mmHg in RP-IOL group, and 25 mmHg in ISF group. Temporary IIOP improved within 2 months with conservative treatment, such as topical β-blockers and carbonic anhydrase inhibitors. There was no difference in mean IOP before and after surgery within the groups and between the groups throughout the follow-up period (Fig. 4).

Changes in postoperative endothelial cell count ( ECC) over time after conventional scleral fixation (C-SF), retropupillary iris-claw intraocular lens (RP-IOL) implantation, and intrascleral fixation (ISF). In all groups, ECC decreased significantly after surgery, but no difference was observed between groups, and remained above 1,700 cells/mm2.
CME occurred in two eyes in C-SF group, one eye in RP-IOL group, and two eyes in ISF group. Most of the CME improved with topical nonsteroidal anti-inflammatory drugs, and one patient in C-SF group and ISF group improved after posterior subtenon triamcinolone acetonide injection. The macular thickness measured by OCT 1 month after surgery was 245.2 ± 52.2 μm in C-SF group, 246.3 ± 71.0 μm in RP-IOL group, and 243.8 ± 55.7 μm in ISF group. In all three groups, the macular thickness slightly increased compared to before surgery at postoperative 1 month, but there was no significant difference between the three groups (Table 2).
No significant anterior chamber inflammation was observed in any of the three groups, and iris atrophy occurred in three eyes in RP-IOL group. Suture exposure occurred in five eyes in C-SF group during the follow-up period, and the mean onset time was 6.5 ± 3.6 months. Among them, scleral patch graft was performed in two eyes. IOL dislocation occurred in five eyes only in RP-IOL group, two of which underwent two reoperations. In all cases, only one side of the haptic was dislocated, and reoperation was performed without any complications with a simple method of pushing the iris tissue into a claw. Complications, such as hypotony, retinal detachment, vitreous hemorrhage, and endophthalmitis, did not occur in any of the three groups.
Discussion
This study is the first to compare the efficacy and safety of C-SF, RP-IOL implantation, and ISF techniques for aphakic eyes or IOL dislocation. Efficacy was evaluated by comparing visual acuity, refractive outcomes such as astigmatism, and PE, while safety was evaluated by comparing ECC loss and postoperative complications.
The mean UCVA and BCVA at 6 months after surgery were similar to those in previous studies of scleral fixation, RP-IOL implantation, and ISF [20,21] and were significantly improved compared to those before surgery; there was no difference according to the surgical methods.
In this study, postoperative ECC was reduced in all three groups, and there was no difference in ECC between the three groups before and 6 months after surgery. ECC loss after surgery can be caused by contact with surgical instruments and IOL during surgery and by the toxic effects of inflammatory mediators [22]. In previous studies, there was no significant difference in postoperative ECC between scleral fixation and RP-IOL implantation and between RP-IOL implantation and ISF; a decrease in postoperative ECC was observed in all three groups [23,24]. The results of this study were consistent with those of previous studies.
RP-IOL group showed significant improvement in BCVA at 1 month after surgery, which was faster than that in the other two groups. This result was similar to that of Hara et al. [25], who compared scleral fixation and RP-IOL implantation for approximately 6 months. Hara et al. [25] reported that the RP-IOL implantation group showed faster visual acuity recovery than scleral fixation group, but there was no significant difference in final visual acuity.
In this study, SE showed a hyperopic shift of 0.56 ± 0.62 D after RP-IOL implantation. A previous study found a statistically significant hyperopic shift in RP-IOL group compared to C-SF group [26]. The results of this study are comparable to those of previous studies. Based on these findings, adjustments for each operator are required, and additional analyses are required by increasing the number of patients in the future to confirm the results.
As for postoperative complications, IOL dislocation occurred in five eyes in RP-IOL group, two of which underwent two reoperations. All were cases of single haptic dislocation, and there were no cases of double haptic dislocation or IOL falling into the vitreous. In one eye, it occurred after face washing, while no trigger was found for the other eye. Iris atrophy was observed in three eyes at the time of dislocation, and the average reoperation time was 7.3 months after surgery. In a previous study, single haptic dislocation occurred in 22 of 225 eyes (9.8%) after an average of 90 days postoperatively; iris atrophy was observed in 59% of disenclavation cases, and disenclavation occurred after face washing in 23% cases [27]. IOL can be reenclavated without any complications in all patients, and postoperative BCVA, SE, and ECC were not significantly affected. Taken together, RP-IOL implantation requires attention to daily life trauma due to the possibility of disenclavation and follow-up for iris atrophic change. However, complications such as anterior chamber inflammation and macular edema were not significant, and it was rare for both haptics to fall out at once; moreover, they can be easily reenclavated.
Based on the results on RP-IOL implantation of this study, iris atrophy or damage may occur during the surgical procedure and is not recommended for patients with a history of glaucoma surgery or flaccid iris. However, even after long-term follow-up, complications such as anterior chamber inflammation and macular edema were uncommon, and postoperative astigmatism was not induced despite the large incision. In addition, the material cost according to the surgical method should be considered. In the case of Artisan, a RP-IOL used in this study, it is made of a nonreimbursable material and can be costly; therefore, it is necessary to decide the surgical method in consideration of the patient’s economic situation as well as the outcome of each surgical method.
ISF has the advantages of shorter operation time, no complications related to suturing, and no additional costs compared to conventional scleral fixation. However, the surgical technique is unfamiliar to the anterior segment surgeon and may take a long time to become skilled in, and the types of IOLs available are limited. Although there were concerns regarding scleritis and haptic slippage, no scleral inflammation or haptic slippage occurred during long-term follow-up, as confirmed by anterior segment photography and tomography in this study.
In this study, SE showed a hyperopic shift of 0.44 ± 1.00 D after ISF. In a previous study by McMillin et al. [28], SE after ISF showed a 0.57 D hyperopic shift compared with the predicted SE using the SRK-T formula, which is consistent with the results of this study.
This study has a limitation in that it is a retrospective analysis of medical records, and patients were not randomly assigned to the different groups. In addition, there is a possibility of selection bias because only cases that could be observed for more than 6 months were included in the analysis to confirm long-term clinical results. Another limitation was that the analysis did not include tilting or decentering of the IOL as factors affecting the postoperative outcomes. Also, postoperative topography was not taken routinely in this study, so it is difficult to confirm postoperative corneal astigmatism and surgically induced astigmatism. Since this study was observed up to 6 months with a small sample size, further studies with larger samples and long-term follow-up of more than 6 months are required.
In conclusion, there were no significant differences in UCVA, BCVA, astigmatism, and ECC loss between C-SF, RP-IOL implantation, and ISF performed in IOL dislocation or aphakic eyes. Although the frequency of IOL dislocation in the RP-IOL implantation group was higher than that in the other groups, it can be reenclavated relatively easily. Both RP-IOL implantation and ISF were as effective as scleral fixation and can be a good alternative to conventional scleral fixation as a treatment for dislocated IOL or aphakic eyes.
Acknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Korean government (the Ministry of Health and Welfare) (No. HI19C0481 and HC19C0142), and a National Research Foundation (NRF) grant funded by the Korean government (the Ministry of Science and ICT) (No. NRF-2021R1C1C1007795) to DHL.