Anterior Ocular Biometrics Using Placido-scanning-slit System, Rotating Scheimpflug Tomography, and Swept-source Optical Coherence Tomography
Article information
Abstract
Purpose
To compare anterior biometry measurements using placido-scanning-slit topography, rotating Scheimpflug tomography, and swept-source optical coherence tomography.
Methods
A retrospective review consisted of 80 eyes of 49 participants who underwent anterior chamber depth (ACD), central corneal thickness (CCT), and keratometry examination on the same day. We used placido-scanning-slit topography (ORBscan II), rotating Scheimpflug tomography (Pentacam HR), and swept-source optical coherence tomography (CASIA SS-1000). The intraclass correlation coefficients and Bland-Altman plots were used to evaluate the agreement and differences between measurements.
Results
The mean ACD values were 2.88 ± 0.43, 2.82 ± 0.50, and 2.68 ± 0.44 mm; and the mean CCT values were 536.96 ± 31.19, 543.79 ± 31.04, and 561.41 ± 32.60 μm; and the mean keratometry (Km) were 43.81 ± 1.69, 43.81 ± 1.77, and 44.65 ± 1.95 diopters; as measured by CASIA SS-1000, Pentacam HR, and ORBscan II, respectively. Among the three devices, ACD was deepest to shallowest in the order of CASIA SS-1000, Pentacam HR, and ORBscan II (p < 0.05). The CCT was thickest to thinnest in the order of ORBscan II, Pentacam HR, and CASIA SS-1000 (p < 0.05). No significant differences in Km values were examined between CASIA SS-1000 and Pentacam HR, whereas ORBscan II overestimated Km with a statistically significant difference compared to the other two devices.
Conclusions
High level of agreement was found between CASIA SS-1000 and Pentacam HR for anterior parameters, including ACD, CCT, and Km, suggesting interchangeability. However, ORBscan II measurements differed considerably with the measurements obtained from the other two devices; therefore, it should not be used interchangeably. However, further studies with repeatability test should be considered in order to elucidate the reliability of each device.
Precise measurements of anterior ocular biometry have gained importance with the development of refractive and cataract surgery and are of great significance for both diagnostic and therapeutic purposes in ocular diseases. Measurement of anterior chamber depth (ACD) is crucial when estimating an accurate calculation of intraocular power in cataract surgery, diagnosing primary angle-closure glaucoma, selecting candidates for phakic intraocular lens implantation, and monitoring the changes in the anterior structures during accommodation [1–3]. Central corneal thickness (CCT) is critical in refractive surgery as a predictive factor for postoperative corneal ectasia, essential for accurate determination of intraocular pressure, as tonometry readings may depend on corneal thickness, and helpful in monitoring corneal conditions [4,5]. In addition, peripheral corneal thickness is known to be a useful diagnostic parameter for identifying corneal pathology, such as keratoconus [6,7]. Keratometry, the radius of the corneal curvature, is another important parameter for corneal examination. Accurate measurements of keratometry are essential for prescribing optimal contact lenses with an appropriate curvature and are important for determining intraocular lens power calculation, as even minor errors tend to result in unexpected refractive outcomes after surgery. Furthermore, mean keratometry (Km) of over 47 diopters (D) is one of the risk factors for developing corneal ectasia after refractive surgery [8]. Likewise, ACD, CCT, and keratometry are anterior ocular parameters that are of great importance in the diagnosis, monitoring of disease, and design of operative plans in patients.
With its growing importance, various optical biometers have been developed to evaluate the anterior segment structure more precisely. In the past, ultrasonic biometry has been regarded as the gold standard for anterior ocular biometry. However, as a contact device, in addition to the risk of creating corneal epithelial defects, considerable variability in measurements is reported due to probe indentation on the cornea and off-the-axis measurements [9]. As a result, noncontact devices are preferred. One of the initial noncontact techniques was based on placido-disk imaging, which uses the reflection of the anterior cornea to estimate the surface corneal curvature [10]. Subsequently, a placido-scanning-slit system (ORBscan II; Bausch & Lomb, Rochester, NY, USA), and a rotating Scheimpflug-based system (Pentacam HR; Oculus, Wetzlar, Germany), which provides multiple images to reconstruct the overall contour of the corneal topography, was introduced [10,11]. A more recent technique to evaluate anterior segment structures is swept-source anterior coherence tomography (CASIA SS-1000; Tomey, Nagoya, Japan). The device uses a scanning beam with a wavelength of 1,310 nm, allowing deeper penetration through opaque corneal layers [12]. With a shorter data acquisition time, it is known to have better repeatability and reproducibility than the Scheimpflug-based device [11–14].
Previous studies have compared anterior segment biometrics, such as ACD and CCT, measured using ultrasonic biometry and noncontact devices [15–19]. Moreover, several recent studies have evaluated the differences and interchangeability between multiple noncontact devices [11,13,20,21]. However, there is a paucity of data to evaluate the agreement of ACD measured with ORBscan II, Pentacam, and CASIA. Therefore, the current study aimed to compare ACD, CCT, and Km data recorded from a cohort of healthy individuals using the ORBscan II, Pentacam HR, and CASIA SS-1000 devices on the same day and evaluate whether the measurements obtained using these devices are interchangeable.
Materials and Methods
Participants
This retrospective observational case series was approved by the Institutional Review Board of Yonsei University College of Medicine (No. 4-2020-1223) and was conducted in accordance with the tenets outlined in the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study. The study included 80 eyes from 49 patients who were referred to Severance Eye Hospital in Seoul, Korea, between June 2020 and December 2020.
Healthy individuals who underwent examination of anterior segment structures with three devices on the same day, in the order of ORBscan II, Pentacam HR, and CASIA SS-1000 were included in the study. The exclusion criteria were as follows: (1) history of ocular trauma, intraocular, or corneal surgery, (2) connective tissue diseases, autoimmune diseases, and other severe systemic diseases that may interfere with ocular health, (3) recent contact lens wear within 4 weeks, and (4) failure to complete all three examinations. Furthermore, patients who were using topical or systemic medications that could affect the iris or angle structure at the time of examination were excluded.
Measurement techniques
All measurements were performed on the same day by a single technician who was skilled at using the three instruments: ORBscan II, Pentacam HR, and CASIA SS-1000. The participant was instructed to sit in a dim room with the chin on the chinrest, the forehead against the forehead bar, and both eyes wide open. Each device was operated according to the manufacturer’s instructions. For each participant, both eyes were measured without the application of any ophthalmic solution, including cycloplegic medication, and in the order of right eye to left. All measurements with each instrument were performed under mesopic conditions in the order of ORBscan II, Pentacam HR, and CASIA SS-1000 between 9:00 and 17:00. An interval of 5 minutes was maintained between each eye within the same device and of 10 minutes between different devices. Only a single scan was performed for each device, but the observer carefully checked the scan quality of each measurement to ensure accurate results.
Data collection
Collected data include age, sex, autorefraction, CCT, ACD, Km, astigmatism keratometry, J0 (Cartesian astigmatism), and J45 (oblique astigmatism) vectors. ACD, which is defined as the distance from the corneal endothelium (posterior corneal surface) to the anterior surface of the lens, was obtained from each device for comparison: ACD (endo) in ORBscan II, ACD (internal) in Pentacam HR, ACD (endo) in CASIA SS-1000 (two-dimensional analysis mode). The investigated indices for CCT included the corneal thickness at the apex (center) of the cornea in all three devices: CCT value at the center of the pachymetry map in ORBscan II, CCT (apex) in Pentacam HR, and CCT (apex) in CASIA SS-1000. To compare the keratometric data among the three devices, anterior keratometry values, in which the radius of the anterior corneal curvature was converted into diopter power using a standardized, fictitious keratometric refractive index of 1.3375, were used: SimK in ORBscan II, Km (cornea front) in Pentacam HR, and AvgK (anterior map) in CASIA SS-1000 [22]. Km was calculated as (steep K + flat K)/2, and the corneal astigmatism was converted to astigmatism vector to J0 as -(steep K - flat K)/2 × cos (2 × axis) and J45 as -(steep K - flat K)/2 × sin (2 × axis).
Instruments
ORBscan II uses a scanning-slit image combined with a placido disc for corneal topography with curvature and pachymetry. The device projects 40 slits, 20 from each side, onto the cornea, and records the backscattered light. After reconstruction of a three-dimensional cornea, the anterior segmental parameters were calculated. Corneal measurements were performed with an acoustic correction factor of 0.94 [23]. To calculate the ACD, the software automatically detects the surface of the corneal endothelium and the anterior surface of the crystalline lens from the obtained scan.
Pentacam HR, a single rotating Scheimpflug camera, is a high-resolution imaging system that uses a monochromatic slit light source at a wavelength of 475 nm. The Scheimpflug camera rotates with the visual axis to take 25 or 50 cross-sectional pictures of the anterior segment. The device calculates a three-dimensional model of the anterior segment from up to 138,000 elevation points, providing information about the anterior and posterior surfaces of the cornea and ACD from the endothelium to the crystalline lens [24].
CASIA SS-1000 is an anterior segment swept-source optical coherence tomography (OCT) device that uses a 1,310-nm light source and produces a scan range of 6.0-mm depth and 16.0-mm diameter. It has a measuring speed of 30,000 axial scans/sec with an axial resolution of ≤10 μm and a transverse resolution of ≤30 μm. The “Corneal Map,” a scan protocol designed to measure corneal thickness and topography, consists of 16 radial B-scans with evenly spaced angular intervals. The collected information was then processed to create a topographic map of the anterior segment [24].
Statistical analyses
Data analysis was performed using the IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). The normal distribution was evaluated using the Kolmogorov-Smirnov test and all corneal measurement values exhibited a normal distribution. Descriptive statistics were determined, including standard deviations, means, medians, and frequencies. Statistical significance was set at p < 0.05. Repeated-measures analysis of variance was performed to compare measurements between devices. The results of three measurements were calculated and presented as mean ± standard deviation. The agreement between different devices was assessed using the Bland-Altman plot method, and intraclass correlation coefficients with 95% limits of agreement were calculated.
Results
A total of 80 eyes from 49 patients were included. The mean age was 61.45 ± 18.10 years (range, 20–86 years). The mean spherical refraction of the patients was −0.56 ± 3.13 D (range, −9.00 to +3.75 D), cylindrical refraction was −1.04 ± 1.11 D (range, −7.75 to 0 D), and spherical equivalence was −1.08 ± 3.29 D (range, −12.13 to 3.38 D).
The mean ACD, CCT, and Km were 2.68 ± 0.44 mm, 561.41 ± 32.60 μm, and 44.65 ± 1.95 D, respectively by ORBscan II; 2.82 ± 0.50 mm, 543.79 ± 31.04 μm, 43.81 ± 1.77 D, respectively by Pentacam HR; 2.88 ± 0.43 mm, 536.96 ± 31.19 μm, 43.81 ± 1.69 D, respectively by CASIA SS-1000. The ACD and CCT measurements obtained from the three systems were sorted from the thickest to the thinnest (ORBscan II > Pentacam HR > CASIA SS-1000, p < 0.05), from the deepest to the shallowest (CASIA SS-1000 > Pentacam HR > ORBscan II, p < 0.05) (Table 1). In corneal power measurements, the Km of ORBscan II was significantly larger than that of Pentacam HR and CASIA SS-1000 (p < 0.05).
A high correlation was observed among the three devices in ACD, CCT, and keratometry (Table 2). The 95% limits of agreement was narrowest between CASIA SS-1000 and Pentacam HR in CCT (−8.642 to −5.01), and narrowest between CASIA SS-1000 and ORBscan II in ACD (0.17 to 0.25) and Km (−1.05 to −0.63). Regarding ACD, the absolute mean difference between measurements was the smallest between CASIA SS-1000 and Pentacam HR (0.06 mm) and the largest between CASIA SS-1000 and ORBscanII (0.21 mm). Regarding CCT, the absolute mean difference between measurements was the smallest between CASIA SS-1000 and Pentcam HR (6.825 mm), and the largest between CASIA SS-1000 and ORBscanII (24.45mm). Regarding Km, the absolute mean difference between measurements was the smallest between CASIA SS-1000 and Pentacam HR (0.01 D), whereas the absolute mean difference between CASIA SS-1000 and ORBscanII (0.84 D) and the difference between ORBscanII and Pentacam HR (0.84 D) showed identical values.
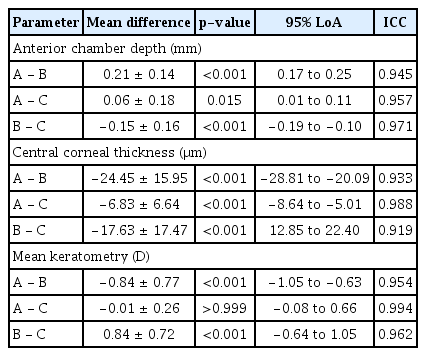
Mean differences, p-values, 95% LoA, and ICC among CASIA SS-1000 (A), ORBscan II (B), and Pentacam HR (C)
All intraclass correlation coefficients were >0.95, except between CASIA SS-1000 and ORBscan II (0.933) and between ORB scan II and Pentacam (0.919). The Bland-Altman plots used to assess the agreement between the devices are shown in Fig. 1A–1I. Overall, ACD,CCT, and keratometry measurements by the three modalities showed a good agreement to one another, but Bland-Altman plots showed better agreement between CASIA SS-1000 and Pentacam HR than with ORBscan II in terms of measuring ACD, CCT, and Km.

Bland-Altman plots show the agreement between the three devices. (A) Central corneal thickness (CCT) between CASIA SS-1000 (CASIA; Tomey, Nagoya, Japan) and ORBscan II (ORB; Bausch & Lomb, Rochester, NY, USA). (B) CCT between CASIA and Pentacam HR (Pentacam; Oculus, Wetzlar, Germany). (C) CCT between ORB and Pentacam. (D) Anterior chamber depth (ACD) between CASIA and ORB. (E) ACD between CASIA and Pentacam. (F) ACD between ORB and Pentacam. (G) Mean keratometry (Km) between CASIA and ORB. (H) Km between CASIA and Pentacam. (I) Km between ORB and Pentacam. The 95% limit of agreement is represented by two solid red lines.
Discussion
Accurate measurements of anterior segment parameters and subsequent precise prediction of the refractive index are essential for satisfactory postoperative visual acuity in cataract and refractive surgery. Furthermore, anterior segment parameters are important in the diagnosis of corneal disease, glaucoma, and other ocular diseases. Therefore, the reliability of the instruments used to evaluate anterior ocular parameters has gained importance in fine-tuning refractive outcomes and identifying ocular diseases. Due to the risk of epithelial defects and possible variability in measurement results due to probe indentation and off-the-axis measurements, noncontact devices are favored today. In this study, we assessed the interdevice agreement of ocular biometric measurements obtained using three different noncontact methods: placido-scanning-slit system (ORBscan II), rotating Scheimpflug imaging-based system (Pentacam HR), and swept-source OCT (CASIA SS-1000). In particular, our study is novel in that no previous study has reviewed and compared the ACD between the three devices previously. The repeatability of each device was not analyzed in this study; however, the high repeatability of each device was comprehensively reported in previous studies [25,26].
In this study, the mean ACD values for the CASIA SS-1000 and Pentacam HR were comparable, whereas the ACD measured by ORBscan II was shallower than the other two methods. Similar to our study, in conventional studies between Pentacam HR and ORBscan II, the ACD measured by Pentacam HR was deeper than ORBscan II [26–28]. However, no study has compared ACD among ORBscan II, Pentacam HR, and CASIA SS-1000.
A study that compared the ACD measured with Pentacam HR, ORBscan II, and Visante OCT reported that the ACD of Visante OCT was deeper than in the ORBscan by 0.1 mm, and shallower than Pentacam HR by 0.04 mm [29]. In another study that compared ACDs of ORBscan, Pentacam HR, and Visante OCT, the mean ACD values were 2.80, 2.93, and 2.98 mm, respectively [28]. Meanwhile, a recent study reported that Visante OCT and CASIA SS-1000 did not show a significant difference between measured ACD values [30].
It is well known that ACD is affected by the accommodative status of the eye [31]. The accommodation process results from ciliary muscle contraction, followed by near-fixation. However, there is no system to prevent this accommodation process during the examination of all devices. Although there is a distant fixation target to minimize the effect of accommodation in each device, the distance and the light stimulus of each target are not identical between the devices, resulting in variability in the outcomes. Furthermore, ACD was automatically measured in Pentacam HR and ORBscan II, whereas in CASIA SS-1000, the examiner manually located the sinus of the angle of the anterior chamber on both sides, and then the software drew a transverse line between them to measure ACD. Although the automatic process in Pentacam HR and ORBscan II may minimize examiner-dependent variations, errors due to fixation loss may interfere with the actual results. These factors may explain the interdevice variation of ACD values. Overall, our results agree with those of the previous studies that ORBscan II measured ACD values to be lower than the other methods, and first investigated the ACD values among the three devices: ORBscan II, Pentacam HR, and CASIA SS-1000.
Previous studies have evaluated the differences in CCT measurements between Pentacam HR and ORBscan II [32]. Lackner et al. [16] and Hashemi et al. [19] reported that t he mean CCT was approximately 30-μm thicker in ORBscan II than in Pentacam HR in normal eyes. Yekta et al. [33] reported that the magnitude of CCT was 16-μm thicker in ORBscan II than in Pentacam HR. However, in a few other studies, the mean CCT was almost the same or thicker in Pentacam HR compared to ORBscan II [26,27,29,34,35]. The mean CCT measured by CASIA SS-1000 was 7 to 8-μm thinner than the mean CCT by ORBscan II [13,36], and 2 to 4.9-μm thicker in Pentacam [24,37].
In this study, the order of measured corneal thickness and the magnitude of differences between each device are consistent with the findings of previous studies in which the CCT measured with CASIA SS-1000 was thinner than that measured with the other two devices. Although all CCT values measured with each device had statistically significant differences compared to the others, CASIA SS-1000 and Pentacam HR presented relatively comparable values with a mean difference of 6.825 ± 6.64 μm, which is not clinically significant enough to make diagnostic or refractive changes.
One of the reasons for the differences in corneal thickness measurement among the three devices may be the different ways of measuring CCT. CASIA SS-1000 takes the respective reflective interfaces of the cornea as the boundary, whereas ORBscan II and Pentacam HR calculate the CCT by measuring the distance between the air-tear film interface and the posterior corneal surface using optical principles of light reflection [38]. Therefore, the CCT values measured by ORBscan II and Pentacam HR may depend on the air-tear film status of each patient, unlike the values measured by CASIA SS-1000. Furthermore, previous studies have reported that ORBscan tends to yield approximately 7% higher CCT values when compared to the “gold standard” of contact ultrasound pachymetry, and in order to compensate its overestimated value, various acoustic correction factors have been introduced, although there is a debate whether it should be applied or not [39]. Our preliminary result, in which corneal thickness measured with ORBscan II showing the highest values among the three devices, is consistent with the previous findings, emphasizing the need for careful interpretation of ORBscan pachymetry data.
Previous studies on keratometric indices using Pentacam HR and CASIA SS-1000 have shown good agreement [11]. Meanwhile, few studies have reported a significant difference in Km between CASIA SS-1000 and Pentacam HR [14,24], but the difference was <0.2 D. In our study, we found no significant differences in Km and other anterior keratometric indices between CASIA SS-1000 and Pentacam HR, but ORBscan II showed significant differences in Km from CASIA SS-1000 and Pentacam HR. The differences in Km from CASIA SS-1000 and Pentacam HR to ORBscan II are consistent with the results reported in the literature, suggesting the interchangeability between Pentacam HR and CASIA SS-1000, but not with ORBscan II [13,26,40]. The identified differences among devices in keratometric indices may be explained by the fact that ORBscan II automatically discards measurements that are considered to be of poor quality without providing comprehensive information. Furthermore, the acquisition time also varies among the instruments, and the longer acquisition time in ORBscan II, which requires a longer period without blinking, may result in tear film evaporation and corneal irregularity [41].
As for the astigmatism keratometry and vector analysis, there were no statistically significant differences in astigmatism keratometry and J45 between devices. However, J0 of Pentacam was smaller than the J0 of CASIA SS-1000 (p = 0.014), whereas no statistical difference was examined between J0 of CASIA SS-1000 and ORBscan II. No previous studies have examined the astigmatism keratometry and vector analysis of the above mentioned devices. Further studies with repeatability test are crucial in identifying irregular astigmatism especially before implanting toric intraocular lens in practice.
There are some limitations to this study. First, a single examiner performed all measurements. Although this may eliminate the risk of inter-observer error, this may also contribute to a bias. Second, our study did not consider the type and severity of cataract, which may have caused variability in the measurements. Third, the study did not include measurements of ultrasound pachymetry, which is considered as gold standard to measure CCT and ACD for many years. Lastly, the study only evaluated patients with healthy corneas with biometric parameters within a relatively normal range. Therefore, the results cannot be applied to patients with pathological alterations or extreme parameters.
In summary, the measurement of ACD was the shallowest by ORBscan II and deepest by CASIA SS-1000, while the CCT was thinnest by CASIA SS-1000 and thickest by ORBscan II. In keratometry, there was no significant difference between CASIA SS-1000 and Pentacam HR, whereas ORBscan II showed a significant difference compared to the other two instruments. Statistically significant differences were found among the three devices in terms of CCT and ACD, whereas in keratometry, there was no significant difference between CASIA SS-1000 and Pentacam HR. However, the mean difference between CASIA SS-1000 and Pentacam HR was minimal in our study (0.01 ± 0.26 D), indicating that the two devices must be used interchangeably. However, ORBscan II showed relatively dissimilar measurements compared to the other two devices and, therefore, should not be used interchangeably. As this is the first study to investigate and compare ACD measurements using ORBscan II, Pentacam HR, and CASIA SS-1000, further studies involving more eyes are necessary to elucidate the interchangeability among the three devices. Furthermore, repeatability test should be considered in order to elucidate the reliability of each device.
Acknowledgements
None.
Notes
Conflicts of Interest: The corresponding author Tae-im Kim has been a member of the Korean Journal of Ophthalmology Editorial Board since 2020. However, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article were reported.
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (No. NRF-2019R1F1A 1061062468).
