Vision-related Quality of Life in Malaysian Children with Threshold and Prethreshold Retinopathy of Prematurity
Article information
Abstract
Purpose
The prevalence of retinopathy of prematurity (ROP) is higher in developing countries compared to developed countries. There is limited data on vision-related quality of life (VRQoL) among children with severe type of ROP in developing countries. This study evaluated the influence of threshold and prethreshold ROP on VRQoL in Malaysian children.
Methods
Multicenter prospective cross-sectional study conducted in three tertiary hospitals in 2018 to 2019. Children less than 7 years old with previous ROP diagnosis were recruited. Patients with systemic comorbidities that affected vision or daily activities were excluded. A parent or guardian completed the Children’s Visual Function Questionnaire (CVFQ) for the assessment of child’s general health, general vision, competence, personality, family impact, and treatment difficulty.
Results
Eight were categorized with threshold ROP, 16 with high-risk prethreshold ROP, and 26 with low-risk prethreshold ROP. Fifty age-matched controls were also included. Mean visual acuity in logarithm of the minimum angle of resolution was 0.46 in the threshold, 0.08 in high-risk prethreshold, and 0.01 in low-risk prethreshold subgroups. Threshold ROP was associated with myopia and strabismus, and associated with poor visual acuity compared to prethreshold ROP. Mean total CVFQ score was significantly lower in the ROP group (p < 0.001) compared to the control group. Mean score and all mean subscale scores were significantly lower in the threshold subgroup compared to high-risk and low-risk prethreshold subgroups, with lowest subscale scores on general vision and general health. There was significant association between gestational age, visual acuity of the better eye, family income, and VRQoL (p < 0.05).
Conclusions
ROP was associated with lower VRQoL in children born prematurely in Malaysia. The threshold ROP group is the most affected. General vision and health domains are their main difficulties encountered. Gestational age, visual acuity of the better eye, and family income affects the VRQoL.
Retinopathy of prematurity (ROP) is a potentially blinding disease that affects immature retinal vasculature in the eyes of premature low birth weight babies. It is a leading cause of childhood blindness, accounting for up to 12% of pediatric vision impairment cases worldwide in developed countries [1–3]. However, the prevalence of ROP among premature children varies markedly by region, averaging 21% in Asian countries, 29.2% to 36.6% among all developed nation, and 11.9% to 58.6% among all developing countries [4–10].
Threshold ROP was defined as ROP of more than 5 contiguous or 8 cumulative clock hours of stage 3 plus ROP in zone 1 or zone 2. While prethreshold ROP was considered as any zone 1 ROP less than threshold, zone 2 stage 2 with plus, zone 2 stage 3 without plus, or zone 2 stage 3 with plus but less than 5 contiguous or less than 8 cumulative clock hours of ROP. Diode laser treatment is the standard treatment for premature babies with threshold ROP. The proportion of premature babies in developing countries that required laser treatment for ROP also varies; for instance, while only 6.5% to 9.3% in Taiwan, and 11.5% in South Korea, 21% in Indonesia, 25.4% in India, and 32.9% in Malaysia require laser treatment [5–8,10].
The majority of children with laser treated threshold ROP have favorable anatomical and visual outcomes. Vision-related quality of life (VRQoL) in children with ROP has been assessed in Brazil and India [11,12], but there are little data from developing countries of South or East Asia according to a recent PubMed search. Such information is critical because more severe types of ROP have been observed in developing countries with limited health resources for neonatal care [13,14]. Further, these data could aid in more efficient allocation of resources [14].
Children’s Visual Function Questionnaire (CVFQ) is a validated and specific instrument designed to measure VRQoL in children up to 7 years of age by parent or caregiver report [15]. Our study aimed to compare the mean total and mean subscales scores (general health, general vision, competence, personality, family impact, and treatment) among children with ROP stratified according to disease severity (threshold, high-risk prethreshold, and low-risk prethreshold).
Materials and Methods
This was a multicenter prospective cross-sectional study conducted in three tertiary hospitals with pediatric ophthalmology services from December 2018 to November 2019: Hospital Universiti Sains Malaysia, Hospital Tengku Ampuan Rahimah, and Hospital Kuala Lumpur. The study was approved by the Research and Ethics Committee of School of Medical Sciences, Universiti Sains Malaysia (No. USM/JEPeM/17110578) and the National Medical Research Registry of Ministry of Health, Malaysia (No. NMRR-17-391-39410). All study protocols were conducted in adherence with the principles of the Helsinki Declaration and Resolution 196/96. Informed consent was obtained from each parent or guardian before participation.
The children and parents or guardians were selected through simple random sampling from the Ophthalmology Pediatric Registry of the participating hospitals. Children aged 7 years old or less with previous history of ROP and born at 32 weeks of gestation or earlier were eligible for the study. The control group was composed of children up to 7 years old and born full-term. The exclusion criteria for children were chronic encephalopathy, hydrocephalus, microcephaly, macrocephaly, central nervous system malformation, intracranial hemorrhage, cerebral visual impairment, and intellectual disability. Children with a history of glaucoma, corneal opacity, nystagmus, microcornea, or amblyopia were also excluded. One parent or guardian of these children participated in this study. Parents or guardians were excluded (with the child) if they refused to answer the questionnaire or had poor English proficiency.
The study was conducted in two phases. In the first phase, the children were recruited according to the final diagnosis of ROP based on the International Classification of Retinopathy of Prematurity [16]. In the second phase, the recruited children with a history of ROP were divided into three subgroups, threshold ROP, high-risk prethreshold ROP, and low-risk prethreshold ROP, as defined by the Cryo-ROP study and Early Treatment Trial of ROP [17,18].
Birth history, gestational age, diagnosis of ROP, and treatment received were obtained from medical records. Educational level and family income were obtained from the parent or guardian. All children in the ROP and control groups had visual acuity and complete ophthalmic assessments. Bilateral visual acuity measures were converted to logarithm of the minimum angle of resolution. Cycloplegic refraction measures were performed by a trained optometrist.
There are two versions of the CVFQ, one for children under 3 years of age and another for children 3 to 7 years of age [15]. The questionnaire for younger children contains 35 items and for older children 40 items on topics related to visual function and VRQoL. Each version has six subscales evaluating general health, general vision, competence, personality, family impact, and treatment difficulty. Subscale scores range from 0 (worst) to 1 (best). The mean score for each subscale was calculated from all subscale questions and the mean total score was calculated as the sum of mean subscale scores. Unanswered questions and “not applicable” responses were omitted from the mean score calculations.
The questionnaire was completed by one parent or guardian during a 30-minute private interview in an enclosed room. The parent or guardian was allowed to ask for clarification on specific items. Instructions were also provided on the first page of the questionnaire. The parent or guardian were instructed to choose a response option for each question or omit the question if the option was not available and/or refused to be answered.
All data were collected and analyzed using the IBM SPSS ver. 24 (IBM Corp., Armonk, NY, USA). All numerical data are expressed as mean ± standard deviation, median ± interquartile range, and categorical data as frequency (%). Mean total CVFQ scores and all subscale scores were compared between ROP and control groups by independent samples t-test and among ROP subgroups by one-way ANOVA. Categorical variables were compared between group and subgroups by Fisher exact test. We used a multiple linear regression model based on the mean total CVFQ score to determine the effect of ROP and other variables (i.e., gestational age, current age, visual acuity of the better eye, family income). A p-value less than 0.05 was considered statistically significant.
Results
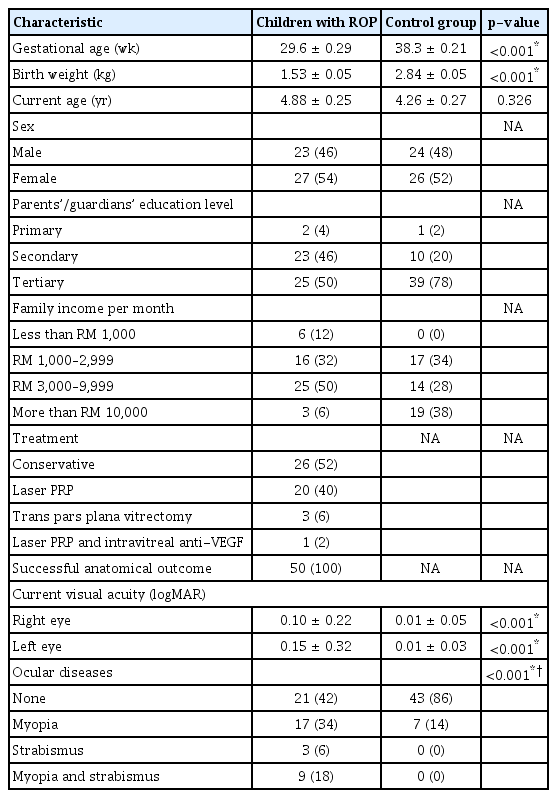
Fifty children with ROP and 50 healthy control children were recruited. Table 1 summarizes demographic and baseline clinical information for both groups. Mean age and sex ratio did not differ between groups. As expected, mean gestational age and birth weight were lower among children with ROP. In addition, the proportion of parents or guardian receiving only primary or secondary education was higher in the ROP group, and there were more low-income families in the ROP group.
Forty percent of the children in the ROP group had received panretinal photocoagulation, 6% pars plana vitrectomy, and 2% had a combination of panretinal photocoagulation and intravitreal injection of antivascular endothelial growth factor. The remaining ROP group children improved spontaneously without intervention. There was a significant difference in mean visual acuity for both eyes between ROP and control group children (p < 0.001). The prevalence of myopia was significantly higher in the ROP group. There were no cases of retinal detachment in either group.
Table 2 presents the distribution of threshold, high-risk prethreshold, and low-risk prethreshold children in the ROP group, and compares the clinical features among these subgroups. None had aggressive posterior ROP. There were significant differences in mean gestational age, mean birth weight, and treatments received among subgroups (p < 0.001). Mean current visual acuity was significantly poorer in the threshold subgroup compared to both prethreshold groups (p < 0.001). The prevalence of myopia was greater in the high-risk prethreshold subgroup than the threshold or low-risk prethreshold group, while strabismus with myopia was highest in the threshold group.
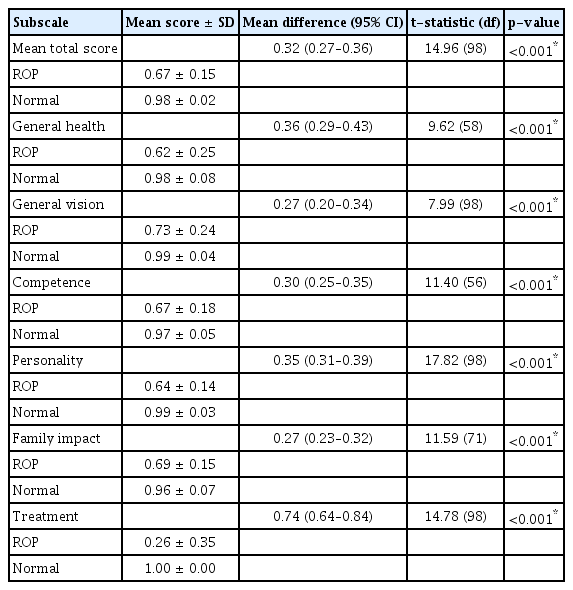
Mean total CVFQ scores and all subscale scores were significantly lower in the ROP group than the control group (p < 0.01) (Table 3). Mean total score and subscale scores also differed significantly among the three ROP subgroups (p < 0.001) with lowest scores in the threshold subgroup and highest scores in the low-risk prethreshold subgroup (Table 4).
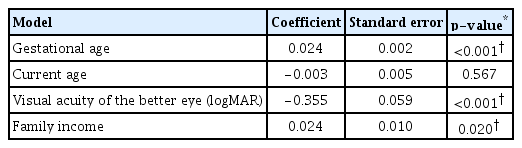
We found significant association between gestational age, visual acuity of the better eye, family income, and mean total score of CVFQ (p < 0.05). Table 5 shows the p-values for each of these variables.
Discussion
Asian infants were reported to develop severe ROP at higher rates than Caucasian infants [19]. In a previous study from Malaysia, 50.1% of premature babies with ROP were classified threshold disease at one of the participating institutions, including 39.3% with stage 3, 3.6% with stage 4, and 7.2% with stage 5 disease [20]. Moreover, 17.4% of the students attending 24 special schools in Malaysia were blind or severely visual impaired as a result of ROP [21].
Our study further documented significantly reduced VRQoL among Malaysian children with ROP according to the CVFQ. This finding is in accord with previous studies in Brazil and India [11,12]. Messa et al. [11] used a different classification system for disease severity (stages 1–3 and stages 4–5), while Kesarwani et al. [12] reported the outcome according to zone (zone I and II) and segregated patients according to age less than 3 years and age 3 years or more.
The mean subscale scores were also consistent with Messa et al. [11]. Mean score in our study ranged from 0.62 to 0.73 among the ROP group but was substantially lower for the items evaluating the effects of treatment on the child and family (mean score, 0.26 ± 0.35). These scores suggest that parents experienced difficulty with treatment (i.e., glasses and eye patches) for the children with ROP. Nevertheless, we were unable to compare treatment subscale with Messa et al. [11] because they did not analyze this component. In contrast, the mean subscale scores between the two different age groups were higher than that of reported by Kesarwani et al. [12].
General vision was the main CVFQ subscale impacted by disease severity, as mean subscale score was markedly lower in children with threshold disease compared to high-risk and low-risk prethreshold disease. Myopia and strabismus were the main causes of this difference, observed in 100% of the threshold group but only 26.8% of the low-risk prethreshold group. In contrast, Kesarwani et al. [12] reported more favorable general vision subscale scores in zone I ROP (mean score, 0.90 ± 0.20) and zone II ROP (mean score, 0.98 ± 0.12), but 88.9% of their patients had good visual acuity (logarithm of the minimum angle of resolution ≥0.3).
Surprisingly, general health was the second most severely affected subscale reported by parents despite excluding candidates with general health issues and chronic systemic illness during recruitment. We suggest that poorer visual acuity with limits participation in sports and other outdoor activities at school and home, which both denies children the associated health benefits. This may be a source of anxiety for the parents as reported by Ozyurt et al. [22] in Turkey. Low educational level and poor socioeconomic status were reported to aggravate the stress experienced by mothers handled children with prematurity [23].
The remaining subscales (competence, family impact, personality, and treatment) exhibited a similar pattern. Vision-related daily activities were less affected in the low-risk prethreshold subgroup while the threshold group was the most affected (p < 0.001), fairly consistent with Messa et al. [11] who compared stages 1–3 ROP to stages 4–5 ROP. However, the children with ROP in our study had poorer visual acuity overall, which could explain why competence, personality, family impact, and treatment subscales were also poorer in the threshold subgroup. The treatment subscale in our study had the least impact on VRQoL among children with ROP. This is possibly because all the centers involved in this study are government hospitals providing low-cost treatment and consultation.
Premature babies achieved lower intellectual, language, memory, and attention abilities compared to term born babies, which contributed to the lower subscales scores [24]. It can be explained by neurophysiological hypothesis postulated by Scher et al. [25], that term babies have fivefold increase in white matter volume and better development, while preterm babies are more vulnerable to remote injury in their brain; thus, it leads to neurocognitive and behavioral deficits.
We observed a significant association between gestational age, visual acuity of the better eye and family income with VRQoL. This data can be justified that by the fact that extremely younger babies require frequent visits to the clinics for ocular examinations especially during the first 2 months of life. Family with poorer outcome will be affected more compared to those with better outcome. Children with ROP who have better visual acuity enjoy a better quality of life.
Differences in VRQoL have been reported between developing and developed countries. Kesarwani et al. [12] reported better competence and personality subscale scores among patients with zone I ROP compared to zone II ROP, while Birch et al. [15] observed significantly better competence, personality, and family impact among American children with ROP and normal visual acuity. Additional studies on VRQoL are warranted in developing countries with more limited treatments, rehabilitation resources, and public services for visually impaired. The major limitation of the present study was absence of the group with similar morbidity. Inclusion of this group will give a better idea and understanding in the future research.
In conclusion, children with threshold ROP were the most severely affected. General vision and health are their main difficulties encountered. These issues merit attention from ophthalmologists, neonatologists, and health policy makers in a developing country.
Acknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: None