Correlation Analysis between Ocular Surface Parameters with Subjective Symptom Severity in Dry Eye Disease
Article information
Abstract
Purpose
To evaluate the clinical symptoms of patients with dry eyes, based on the ocular surface disease index (OSDI) and analyze the relationship between OSDI and various ocular surface parameters.
Methods
This was a retrospective study that included 45 eyes of 45 dry eye patients who visited the Seoul Nune Eye Hospital from August 2017 to December 2017. The patients were assessed by non-invasive keratography for the first break-up time, lipid layer thickness (LLT), tear osmolarity, tear matrix metalloproteinase-9 immunoassay as well as with the conventional Schirmer I test and fluorescein break-up time. The patient's symptoms were evaluated by the OSDI questionnaires and correlations were analyzed based on the parameters described above.
Results
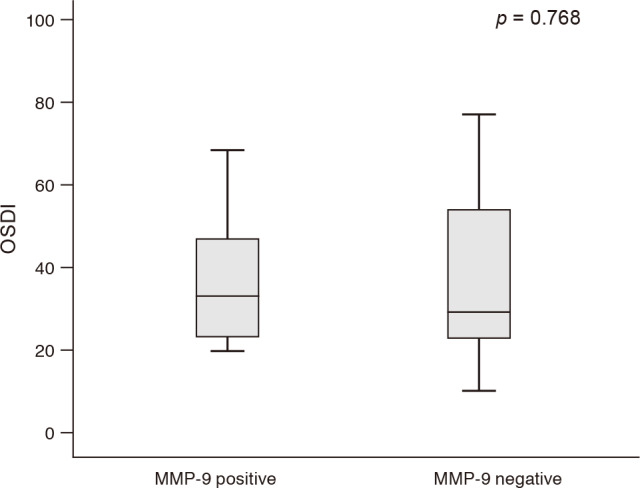
There were significant negative correlations between OSDI and non-invasive keratography for the first break-up time (p = 0.038, r = −0.330), and LLT (p = 0.005, r = −0.426). However, there were no significant correlations between OSDI and fluorescein break-up time, Schirmer I score, and tear osmolarity (p = 0.173, 0.575, and 0.844 respectively). OSDI was not significantly different between matrix metalloproteinase-9 positive and negative groups (p = 0.768).
Conclusions
Non-invasive examinations such as non-invasive keratograph break-up time and interferometry of LLT can be efficient tools for evaluating dry eye symptoms.
Dry eye is a multifactorial disease related to impaired tear film homeostasis and is accompanied by tear film instability and hyperosmolarity, and ocular surface inflammation [1]. One characteristic feature of dry eye disease is the discordance between the patient's symptoms and clinical signs, which makes dry eye disease treatment more challenging [2]. As part of continuing efforts to minimize this gap between symptoms and signs, various emerging technologies have been developed such as a tear matrix metalloproteinase-9 (MMP-9) immunoassay, the tear osmolarity test, and some diagnostic imaging methods, including corneal topography, ocular coherence tomography, meibography, and interferometry, as well as conventional tear break-up time (TBUT), the Schirmer test, and an ocular surface staining score. Moreover, these testing methods have become more accessible and are already widely used.
Several previous studies evaluated the correlation between the ocular surface disease index (OSDI) and objectively quantifiable parameters in dry eye patients [34]. They examined dry eye parameters including conventional fluorescein TBUT (FBUT), the Schirmer test, cornea and conjunctival staining score, blepharitis severity grade, and/or lipid layer thickness (LLT) by Lipiview (TearScience, Morrisville, NC, USA), to evaluate correlations between results and OSDI. They concluded that meibomian gland dysfunction should be an important factor that influences subjective pain and discomfort in dry eye patients. However, to our knowledge, no studies have evaluated additional further ocular surface parameters, including the tear MMP-9 immunoassay using InflammaDry (RPS Diagnostics, Sarasota, FL, USA), tear osmolarity using the TearLAB Osmolarity system (TearLab Corporation, San Diego, CA, USA), and the non-invasive keratograph TBUT using Keratograph 5M (Oculus, Wetzler, Germany), in addition to the parameters outlined above. We examined almost all ocular surface parameters using appropriate techniques to analyze the correlation between OSDI and these parameters.
Materials and Methods
A chart review of the 45 patients who presented at the Seoul Nune Eye Hospital between August 2017 to December 2017 due to dryness in their eyes and were diagnosed with dry eye disease was performed. The diagnosis of dry eye disease was made when the patients experienced dry eye symptoms and the FBUT was shorter than 10 seconds and/or cornea or conjunctival superficial erosion was seen in slit-lamp examination by the same examiner (THC). We excluded patients with conditions that could affect dry eye parameters such as contact lens use, history of refractive, glaucoma, or retina surgery, cataract surgery within 6 months, systemic disease including diabetes, autoimmune disease, cranial nervous system disorders, and use of ocular medications other than artificial tears. Approval from the institutional review board of Nune Eye Hospital was obtained (N-1802-002-999) and informed consent was waived because of the retrospective nature of the study.
Forty-five subjects were examined with non-invasive keratograph first break-up time (NIKf-BUT), LLT, tear osmolarity, tear MMP-9 immunoassay, FBUT, and Schirmer I score assessments. After several blinks, the NIKf-BUT, which is determined as the time between the blink and the first mire distortion, was measured by Keratograph 5M. A Lipiview provides data for the minimum, maximum, average LLT, and partial blink rate. The average LLT was used in this study because the average LLT provided a more appropriate representation of tear film stability than minimum or maximum LLT [5]. A tear sample from the patient's palpebral conjunctiva was collected and the osmolarity was measured with the TearLAB Osmolarity system. An InflammaDry tear immunoassay test was performed to detect tear MMP-9. A sampling fleece was used to collect tears by gently dabbing multiple locations of palpebral conjunctiva, then a sample collector was assembled onto the test cassette and the test was interpreted after 10 minutes had elapsed. The presence of one blue line and one red line, regardless of the intensity of the red line in the test's result window, was regarded as a positive test result. The FBUT was measured as the time interval between fluorescein staining and the appearance of the first dry spot using the cobalt blue filter. A Schirmer I test was performed without topical anesthesia by placing the standardized strip in the lower lid temporal side for 5 minutes. Thirty-minute intervals between all measurements ensured subsidence of effects for each examination. Only the right eye was studied in this current study. OSDI (Allergan, Irvine, CA, USA) questionnaires were used to assess patient dry-eye symptoms.
Continuous variables were expressed as mean ± standard deviation. Pearson correlation analysis was used to evaluate correlation between the OSDI and ocular surface parameters. An independent t-test was used to compare the OSDI between the MMP-9 positive group and negative group. In all statistical tests, a p-value < 0.05 was considered statistically significant. Data was analyzed using PASW Statistics ver. 18 (SPSS Inc., Chicago, IL, USA).
Results
The mean age of the patients was 51.5 ± 14.4 years and 36 of 45 patients were female. The mean FBUT was 5.2 ± 2.3 seconds, the mean Schirmer I score was 12.7 ± 9.1 mm, and the mean OSDI was 38.3 ± 20.1. The demographic characteristics and dry eye parameters are summarized in Table 1. All patients were considered level 1 or 2 based on the Korean Corneal Disease Study Group guidelines for the diagnosis of dry eye [6].
Among parameters that included the FBUT, Schirmer I score, NIKf-BUT, LLT, tear osmolarity, and OSDI, OSDI showed significant negative correlations with NIKf-BUT (p = 0.038, r = −0.330) and LLT (p = 0.005, r = −0.426) (Table 2 and Fig. 1A, 1B). Other dry eye parameters such as FBUT, Schirmer I score, and tear osmolarity did not show significant correlations with OSDI (p = 0.173, 0.575, 0.844, respectively) (Table 2 and Fig. 1C–1E). There was also a positive correlation between NIKf-BUT and LLT (p = 0.024, r = 0.353) (Fig. 1F). The OSDI was significantly higher in the below-average LLT group (LLT ≤77.8, mean LLT was 77.8 ± 17.8 nm) than in the above-average group (LLT >77.8) (p = 0.002) (Fig. 2). The mean OSDI was 40.1 ± 19.0 in the MMP-9 positive group and 38.2 ± 20.9 in the MMP-9 negative group. There was also no significant difference in the OSDI of the MMP-9 positive group and MMP-9 negative group (p = 0.768) (Fig. 3).

Pearson correlation scatter plot for (A) non-invasive keratograph first break-up time (NIKf-BUT) and ocular surface disease index (OSDI), (B) lipid layer thickness (LLT) and OSDI, (C) fluorescein break-up time (FBUT) and OSDI, (D) Schirmer I score and OSDI, (E) tear osmolarity and OSDI, (F) LLT and NIKf-BUT. (A), (B), and (F) showed significant correlations: (A) r = −0.330, p = 0.038; (B) r = −0.426, p = 0.005; (F) r = 0.353, p = 0.024.

Comparison of the ocular surface disease index (OSDI) between the above-average lipid layer thickness (LLT) group and below-average LLT group. The mean average LLT was 77.8 ± 17.8 nm. A p-value was calculated by independent t-test.
Discussion
Impaired tear homeostasis, which is related to the amount of tears produced, the rate of tear evaporation, goblet cell density, and the presence or absence of inflammation, can lead to dry eye [7]. Dry eye diseases have typically been evaluated by patient's subjective symptoms, tear production, tear film instability, and damaged cornea and conjunctival status [6]. Previous studies have investigated parameters that could be associated with subjective discomfort of dry eye [34]. In contrast with expectations, the studies revealed that TBUT, which is related to tear film instability, was not significantly correlated with OSDI. The TBUT was measured by fluorescein dye instillation in their studies and in this study, and the FBUT was not associated with OSDI. However, NIKf-BUT was significantly correlated with OSDI in this study (p = 0.038, r = −0.330). This is because a non-invasive examination would exclude fluorescein's interference in tear stability and could be measured with more consistency. Moreover, non-invasive keratograph break-up time (NIKBUT) was associated with greater repeatability in dry eyes than in non-dry eyes because non-dry eyes need to be opened longer during examination due to longer TBUT which induces more reflex tear secretion [8].
FBUT is a widely-used conventional diagnostic method for tear film instability. However, there are some limitations to FBUT because fluorescein can destabilize the tear film and measurements can be affected by examiners [9]. The Keratograph 5M can measure NIKBUT by recording a real time image of the entire tear break-up course and measure the time between the blink and the mire distortion. Twenty-two placido rings are reflected to the cornea and their distortions are analyzed within 192 divided sections and visualized by a color-coded map. The timing of the first break in the tear film, NIKf-BUT and the average time of all the break-ups during the measurement (non-invasive keratograph average break-up time) are displayed. NIKf-BUT was used in this study and the mean NIKf-BUT was 5.8 ± 2.0 seconds, which was longer than the FBUT, which was 5.2 ± 2.3 seconds. However, previous studies showed that the NIKBUT is shorter than the FBUT because software analysis of NIKBUT may detect very early tear film changes and heat from the LED (light emitting diode) may induce more tear break-ups [81011]. In this study, the examiner subjectively measured the FBUT and did not use a stopwatch, which might have influenced these conflicting results.
LLT was also significantly correlated with the OSDI (p = 0.005, r = −0.426). Blackie et al. [12] also revealed that LLT was significantly correlated with standard patient evaluation of eye dryness. However, Seo et al. [3] and Kim et al. [5] found that LLT was not correlated with OSDI. Whether the LLT is associated with OSDI remains controversial because different study designs or patient populations have reported conflicting results, however, the blepharitis severity score [4] or quality of the meibum [3] was significantly correlated with the OSDI. Meibomian gland dysfunction, one of the most important factors of dry eye, promotes tear film evaporation and decreases TBUT [13]. Several previous studies showed that TBUT and LLT are significantly correlated [5141516], and our study also confirmed that there was a significant association between NIKf-BUT and LLT (p = 0.024, r = 0.353). Insufficient LLT associated with tear film instability might result in patient discomfort with a high OSDI.
Other ocular surface parameters such as the Schirmer I score, tear osmolarity, and tear MMP-9 presence were not associated with OSDI. Because this study excluded autoimmune disease patients and the mean Schirmer I score was relatively high at 12.7 ± 9.1 mm, the Schirmer I score might not show significant correlations with OSDI. MMP-9 is an ideal inflammation marker because it is induced by all of the primary mediators, including IL-1β, TNF-α, IL-6, IL-8, IL-17 and accumulates throughout the inflammatory cascade [171819]. MMP-9 destabilizes tear film, contributes to corneal barrier dysfunction which causes epithelial cell desquamation, and results in pain and fluctuating vision [19]. However, the results of our study and other studies have indicated that there is no significant difference in the symptoms and signs of dry eye patients between MMP-9 positive and negative groups [20]. Hyperosmolarity of the tear film has been proposed as one of the key pathogenic factors in dry eye disease, but a high variability of measurements and specific cut-off values to diagnose dry eye have not been established. Correlations bet ween tear osmolarity and OSDI are currently controversial [2122].
There were several limitations to this study. Performing multiple non-invasive examinations such as NIKBUT and interferometry for LLT, as well as invasive examinations such as the Schirmer I test, FBUT, tear osmolarity, and MMP-9 immunoassay in one day could affect the test measurements, in spite of interval times between each examination. In addition, this study did not analyze the subjects based on dry eye severity because only relatively mild dry eye participants were enrolled. Gross examinations such as the cornea and the conjunctival staining score, blepharitis severity, or meibum quality were not evaluated as parameters. Due to small number of patients who showed cornea or conjunctival superficial erosions, results were not available for analyzing the association between OSDI and the ocular surface staining score. Despite these limitations, this study analyzed almost all ocular surface parameters that can be examined recently and determined whether OSDI was associated with NIKf-BUT or LLT, and also found that NIKf-BUT and LLT were significantly correlated with each other. The results of this study indicate that these non-invasive parameters could explain patient discomfort, which can influence clinicians to select appropriate clinical management strategies. Moreover, because these parameters can be visualized, patients can easily recognize their conditions and might feel validated when discussing concerns with their providers.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.