Efficacy of Topical Cyclosporine Nanoemulsion 0.05% Compared with Topical Cyclosporine Emulsion 0.05% and Diquafosol 3% in Dry Eye
Article information
Abstract
Purpose
To evaluate the efficacy and safety of cyclosporine nanoemulsion 0.05% compared to cyclosporine emulsion 0.05% and diquafosol sodium 3%.
Methods
This was a multicenter, randomized, evaluator-masked, active control, parallel, phase IV study. A total of 227 patients were randomly allocated to instill cyclosporine nanoemulsion 0.05% (CN) twice daily, cyclosporine emulsion 0.05% (CE) twice daily, or diquafosol sodium 3% (DQ) six times daily. Non-inferiority of CN was analyzed by primary endpoint (cornea and conjunctival staining scores at week 12). The secondary endpoints were scores of corneal staining, conjunctival staining, tear break-up time, Schirmer test, and Ocular Surface Disease Index at weeks 4 and 12.
Results
Primary endpoints showed statistically significant improvements in all groups. Primary endpoints were −6.60 for the CN group, −5.28 for the CE group, and −6.63 for the DQ group (National Eye Institute scale from 0 to 33), verifying the non-inferiority of CN compared to CE (95% confidence interval, −0.15 to 2.80, Δ>−2.88). In intergroup comparison between CN and CE groups, the CN group had significantly more decreased conjunctival staining score at week 12. Intergroup comparison between CN and DQ groups showed consistent statistically significant improvements in TBUT and Schirmer test in the CN group. In the DQ group, TBUT showed late statistically significant improvement at week 12 and Schirmer test showed relatively short-term statistically significant improvement at week 4.
Conclusions
Cyclosporine nanoemulsion 0.05% was equivalently efficient compared to cyclosporine emulsion 0.05% and diquafosol sodium 3%. In addition, CN showed significant improvements in several parameters for treatment of dry eyes.
Current management of dry eye disease includes tear supplementation by artificial tears or lubricants, tear stimulation, anti-inf lammatory agents, immunomodulatory agents, and environmental strategies [1]. Cyclosporine, an immunomodulatory agent, is a calcineurin inhibitor that impedes activation of T lymphocytes, ultimately reducing inf lammatory reactions and apoptosis [23], or programmed cell death [4]. Because cyclosporine has a very high molecular weight and is hydrophobic [56], it has very poor (20–30 µg/mL) solubility in water [7]. To improve this limitation for an ophthalmic solution, 0.05% cyclosporine anionic emulsion formulation (Restasis; Allergan, Irvine, CA, USA) was developed as a mixture of immiscible components and surfactants (castor oil, glycerin, polysorbate 80) [89]. This has been available as a Food and Drug Administration-approved treatment for dry eye disease since 2003. However, the dispersed particle size of the emulsion formulation is relatively large and diversely distributed, ranging from 50 to 1,000 nm [6]. The emulsion fluid is turbid, thermodynamically unstable, and readily separated into two immiscible liquid phases, resulting in flocculation, sedimentation, creaming, and coalescence [9]. Moreover, adherence tends to be decreased because of burning and stinging sensations [10]. To overcome these limitations of emulsion formulation, nanoemulsion technology has been introduced to manufacture a novel cyclosporine ophthalmic solution. The particle size of the nanoemulsion ranges from 10 to 100 nm, smaller than that of an emulsion, thereby maintaining optical transparency. Nanoemulsion formulation is considered a thermodynamically stable liquid dispersion that provides improved bioavailability and efficacy of lipophilic drugs [1112]. A novel 0.05% cyclosporine ophthalmic nanoemulsion (CN; Cyporin N, Taejoon, Seoul, Korea) was commercially developed.
Diquafosol sodium is a P2Y2 receptor agonist that promotes tear fluid section from conjunctival epithelial cells and mucin secretion from conjunctival goblet cells [131415]. Diquafosol improves the stability of tear films and hydration of the ocular surface. Diquafosol sodium ophthalmic solution 3% (DQ; Diquas, Santen, Osaka, Japan) is currently approved in Korea and Japan for treatment of dry eye [16]. In randomized, double-masked, multicenter trials, diquafosol ophthalmic solution has been shown to be equal to sodium hyaluronate ophthalmic solution 0.1% based on fluorescein staining score [17]. However, no report has compared the efficacy of these three types of commercially available eye drops. In this clinical trial, the efficacy and safety of CN was evaluated compared to cyclosporine ophthalmic emulsion 0.05% (CE) and DQ for dry eye disease.
Materials and Methods
Study population
This study was conducted in accordance with the ethical principles specified in the Declaration of Helsinki and Good Clinical Practice Guidelines. The study protocol and informed consent were reviewed and approved by the institutional review board (XC16MIMV0056S) before study initiation. Written informed consent was obtained from each patient before the start of this study. Power analysis was performed to justify the number of patients enrolled in the study. The study was conducted at multiple clinical sites. This trial was registered in the Current Research Information System (http://cris.nih.go.kr) and World Health Organization International Clinical Trials Registry Platform (https://www.who.int/ictrp). The trial registration number is KCT0002180.
Study design
This is a multicenter (12 centers), randomized, evaluator-masked, active control, parallel, non-inferiority, phase IV study. A total of 228 patients with dry eye disease who underwent a screening test were enrolled. The 227 eligible patients were randomly allocated to receive CN, CE, or DQ. Patients in the CN group instilled CN (Cyporin N) twice daily. Patients in the CE group instilled CE (Restasis) twice daily. Patients in the DQ group instilled DQ (Diquas) six times daily. To prevent bias from differences in the total number of eye drops per day among these groups, patients in the CN and CE groups were asked to instill 0.15% hyaluronic acid (New Hyaluni Ophthalmic Solution 0.15%, Taejoon) four times daily. If possible, the total number of eye drops should not exceed six per day. Even if eye drops were instilled six times a day, a minimum amount of 0.15% hyaluronic acid could be added when patients felt discomfort. Patients were examined at week 4, week 8, and week 12 after treatment initiation. At week 4 and week 12, efficacy and safety were evaluated. At week 8, adherence and safety were evaluated without evaluating drug efficacy.
Study population
Adult patients (19 years or older) were eligible for participation if they had a diagnosis of dry eye disease according to the following criteria: (1) cornea fluorescein staining ≥4 National Eye Institute (NEI) scale and (2) tear break-up time (TBUT) ≤10 seconds. Exclusion criteria were: (1) patients who used cyclosporine or diquafosol systemically or topically within 4 weeks before the screening period; (2) patients who used topical agents to treat another ocular disease (glaucoma, allergy, infection, etc.) within 4 weeks before the screening period; (3) patients who used any drugs that might influence the state of dry eye within 4 weeks before the screening period; (4) patients with Sjogren syndrome; (5) patients who needed to use contact lenses during the period of study; (6) patients with eyelid disease (trichiasis, entropion, etc.) or anterior ocular disease (herpes keratitis, cicatricial pemphigoid, pterygium, neurotrophic keratitis, keratoconus etc.) who underwent ocular operation (punctal plug or nasolacrimal drainage process) within 4 weeks before the screening period; and (7) patients with hypersensitivity to drugs or who were pregnant.
A total of 227 patients who passed the screening test were randomly assigned to each group (76 patients in the CN group, 74 patients in the CE group, and 77 patients in the DQ group). The safety population (216 patients) who instilled the received ophthalmic solution at least once included 71 patients in the CN group, 72 patients in the CE group, and 73 patients in the DQ group. The full analysis set population (190 patients) who instilled at least one dose of the received ophthalmic solution and provided data to evaluate the first efficacy endpoint included 62 patients in the CN group, 65 patients in the CE group, and 63 patients in the DQ group. The per protocol set population (173 patients) who completed the period of treatment included 58 patients in the CN group, 58 patients in the CE group, and 57 patients in the DQ group (Fig. 1).
Randomization
Subjects were randomized and equally assigned to three groups through stratified block randomization and sequentially allocated at each site through an interactive web-based response system. The randomization results were partially double-masked during the full study period. All medications were provided after packing with an aluminum pouch to maintain the double-masked condition because medications had different colors and bottle shapes.
Assessment of outcome measure
1) Efficacy assessment
Changes in the corneal and conjunctival staining scores from baseline and at week 12 served as the primary efficacy endpoints of this trial. Secondary efficacy endpoints included corneal and conjunctival staining scores at week 4, corneal staining scores at weeks 4 and 12, conjunctival staining scores at weeks 4 and 12, and TBUT at weeks 4 and 12. The results of the Schirmer test at weeks 4 and 12 were used as objective values, while Ocular Surface Disease Index (OSDI) scores at weeks 4, 8, and 12, as well as satisfaction and adherence, were used as subjective values.
According to the National Eye Institute/Industry Workshop report [18], corneal and conjunctival staining was evaluated under a slit lamp microscope with a cobalt blue filter (scale from 0 to 33). The cornea was divided into five sections: center, nasal, temporal, superior, and inferior. While the patient blinked normally, 5 µL of 2% fluorescein solution were instilled in the conjunctival sac. Fluorescein was scored based on 0 to 3 points of the NEI scale at each section (scale from 0 to 15). The conjunctiva was divided into six sections: three sections at the nasal side and three sections at the temporal side. Then, 20 µL of 1% lissamine green solution was instilled in the conjunctival sac. Conjunctival staining was evaluated under low illumination and scored based on 0 to 3 points of the NEI scale at each section (scale from 0 to 18).
To assess TBUT, the elapsed time from a normal blink to the first appearance of a dry spot in the tear film was measured in a centisecond unit after corneal staining with 5 µL of 2% fluorescein solution. The average elapsed time was calculated after three repeated measurements.
Lacrimal function was evaluated for the Schirmer test, including physiologically basic and reflective lacrimal secretion. Without anesthesia, the Schirmer test strip was placed on the temporal 1/3 of the lower eyelid between the lower palpebral conjunctiva and the lower bulbar conjunctiva. After 5 minutes, the length of the absorbed tear fluid on the strip was measured in millimeters.
To assess instillation adherence, all patients were instructed to record the number of drops they instilled for both the investigational drug and lubricant daily on the ‘Patient Diary’ and to return the records at each visit.
Satisfaction with these trial drugs was assessed and compared through a survey addressing the sensation of using eye drops, patient preference, and improvement in symptoms at the end of the trial. Sensation was classified as overall satisfaction, burning, stinging, blurring, stickiness, smoothing, and moisturizing.
2) Safety assessment
The safety variable was the occurrence of adverse events determined at various visits based on physical signs and symptoms, external eye examination, slit-lamp microscopy, visual acuity, intraocular pressure, and funduscopy.
3) Satisfaction assessment
At every visit, participants were asked to complete a questionnaire and a visual analog scale (from 0 to 10). The questionnaire was composed of five questions about satisfaction with study drugs (overall satisfaction, burning/stinging, blurring, stickiness, and smoothing/moisturizing).
Statistical analysis
All statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA). To assess primary and secondary endpoints, changes from baseline were analyzed using Wilcoxon signed-rank tests. Intergroup comparison was performed using Wilcoxon rank-sum tests between the two groups and Kruskal-Wallis tests among the three groups. Non-inferiority of the CN group compared to the CE group was determined if the 95% confidence interval (CI) for the intergroup difference was calculated and the lower limit of CI exceeded an inferiority margin of −2.88. To assess safety, the frequencies or ratios of variables were measured, and intergroup differences were analyzed using the chi-square test.
Results
Participant characteristics
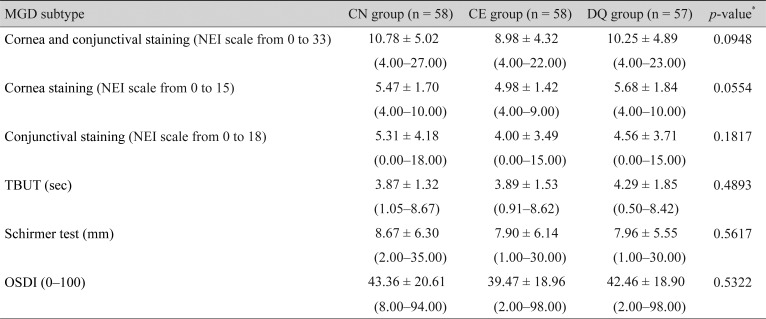
No statistically significant differences were observed in sex, age, past history (within 6 months), surgical history (within 6 months), or present illness among the three groups. Although patients were randomly allocated to three groups, baseline corneal and conjunctival staining scores in the CN group were significantly (p = 0.0396) higher than those in the CE group. Therefore, severity of dry eye disease was significantly worse in the CN group than in the CE group. However, no statistically significant difference was observed in the baseline corneal and conjunctival staining score between the CN and DQ groups and in a comparison among the three groups (Table 1).
Efficacy evaluation
1) Primary endpoint
Changes in cornea and conjunctival staining scores from baseline to week 12 were −6.60 ± 4.47 in the CN group, −5.28 ± 3.47 in the CE group, and −6.63 ± 4.72 in the DQ group. All groups showed statistically significant improvements (all p < 0.0001). No statistically significant difference was found between the two groups or among the three groups. In a non-inferiority test of the CN and CE groups, the lower limit of the 95% CI for intergroup differences was −0.15, which was above the non-inferiority margin of −2.88. This verified the non-inferiority of CN to CE (Table 2).
2) Secondary endpoints
Changes in corneal and conjunctival staining scores from baseline to week 4 were −4.74 ± 4.63 in the CN group, −3.28 ± 4.18 in the CE group, and −4.04 ± 4.12 in the DQ group, respectively. All groups showed statistically significant improvements (all p < 0.0001). No statistically significant difference was found between the two groups or among the three groups in all periods.
Changes in corneal fluorescein staining score from baseline to weeks 4 and 12 were −2.50 ± 2.18 and −3.59 ± 2.24 in the CN group, −2.09 ± 1.76 and −3.45 ± 1.48 in the CE group, and −2.19 ± 2.42 and −3.82 ± 2.09 in the DQ group, respectively. All groups showed statistically significant improvements (all p < 0.0001). No statistically significant difference was observed between the two groups or among the three groups in all periods (Fig. 2A).

Secondary endpoints. (A) Changes in corneal fluorescein staining score from baseline (National Eye Institute [NEI] scale from 0 to 15). *p < 0.05. (B) Changes in conjunctival lissamine green staining score from baseline (NEI scale from 0 to 18). *p < 0.05. (C) Change in tear break-up time (TBUT) from baseline. *p < 0.05. (D) Changes in Schirmer test score from baseline. *p < 0.05. (E) Changes in Ocular Surface Disease Index (OSDI) score from baseline (OSDI score from 0 to 100). *p < 0.05.
Changes in conjunctival lissamine staining score from baseline to weeks 4 and 12 were −2.24 ± 3.40 and −3.02 ± 3.38 in the CN group, −1.19 ± 3.05 and −1.83 ± 2.91 in the CE group, and −1.84 ± 2.93 and −2.81 ± 3.47 in the DQ group, respectively. All groups showed statistically significant improvements (week 4, p ≤ 0.0004; week 12, p < 0.0001). A comparison between the CN and CE groups and among the three groups showed statistically significant differences at week 12 (p = 0.0235 and p = 0.0384, respectively). A comparison between the CN and DQ groups showed no statistically significant difference in all periods (Fig. 2B).
Changes in TBUT from baseline to weeks 4 and 12 were 0.77 ± 1.78 and 1.69 ± 2.45 in the CN group, 0.63 ± 2.24 and 1.29 ± 2.97 in the CE group, and 0.17 ± 1.95 and 0.73 ± 2.43 in the DQ group, respectively. Statistically significant improvements were shown in TBUT in the CN and CE groups at week 4 (p = 0.0034 and p = 0.0364, respectively). Improvements continued until week 12 (p < 0.0001 and p = 0.0006, respectively). However, in the DQ group, a relatively late significant improvement was observed at week 12 (p = 0.0281). A comparison between CN and CE, CN and DQ, and among the three groups showed no statistically significant difference in all periods (Fig. 2C).
Changes in Schirmer test score from baseline to weeks 4 and 12 were 0.83 ± 5.26 and 1.47 ± 6.20 in the CN group, 1.68 ± 4.88 and 2.63 ± 5.94 in the CE group, and 1.56 ± 5.45 and 1.06 ± 6.32 in the DQ group, respectively. Statistically significant improvements were observed at week 4 in all groups (p = 0.0418, p = 0.0202, p = 0.0168, respectively). However, at week 12, only the CN and CE groups showed statistically significant improvements ( p = 0.0031, p = 0.0005, respectively). This statistically significant improvement was not observed in the DQ group at week 12. No statistically significant difference was found between the groups or among the three groups in all periods (Fig. 2D).
Changes in OSDI score from baseline to weeks 4, 8, and 12 were −11.88 ± 18.18, −11.28 ± 17.60, and −13.03 ± 19.63 in the CN group, −12.17 ± 19.68, −14.76 ± 18.38, and −12.98 ± 18.29 in the CE group, and −15.72 ± 15.85, −14.84 ± 19.58, and −16.11 ± 20.87 in the DQ group, respectively. Statistically significant improvements were observed in all groups at weeks 4, 8, and 12 (all p < 0.0001). No statistically significant difference was found between the groups or among the three groups in all periods (Fig. 2E).
Instillation adherence
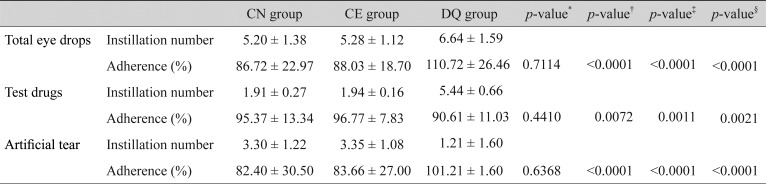
Total instillation adherence rates (6 times per day) were 86.72 ± 22.97% in the CN group, 88.03 ± 18.70% in the CE group, and 110.72 ± 26.46% in the DQ group. Instillation adherence of the DQ group was higher than that of the CN group (p < 0.0001). However, the instillation adherence rate of the test drug (twice per day in CN and CE groups, 6 times per day in DQ group) showed opposite results (95.37 ± 13.34% in the CN group, 96.77 ± 7.83% in the CE group, and 90.61 ± 11.03% in the DQ group). The instillation adherence of the CN group was significantly higher than that of the DQ group (p = 0.0072). No statistically significant difference was observed in instillation adherence between the CN and CE groups (Table 3).
Instillation satisfaction
Overall satisfaction in the CN group was significantly (p = 0.0038) superior to that in the CE group, and there was a superior tendency (p = 0.0543) compared to the DQ group. Burning and stinging sensations were reduced in the CN group compared to the CE group (p = 0.0639). However, no statistically significant difference was observed in burning and stinging sensations between the CN and DQ groups. Stickiness sensation in the CN group was significantly greater than that in the CE or DQ group (p = 0.0124, p = 0.0454, respectively). However, blurring, smoothing, and moisturizing were similar among the three groups.
Safety evaluation
In the safety population, 56 adverse events occurred in 32 (14.81%) patients, including 21 cases in 11 patients (15.49%) of the CN group, 20 cases in 11 patients (15.28%) of the CE group, and 15 cases in 10 patients (13.70%) of the DQ group. Specifically, 11 ocular adverse events occurred in 6 patients (2.78%): five cases occurred in two patients (2.82%) of the CN group, three cases occurred in one patient (1.39%) of the CE group, and three cases occurred in three patients (4.11%) of the DQ group. No statistically significant intergroup difference was shown. Ocular adverse events included ocular pain, irritation, foreign body sensation, and conjunctivitis. No serious adverse event was observed in this trial.
Discussion
According to the definition described by the Tear Film and Ocular Surface Dry Eye Workshop II in 2017, dry eye is a multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiologic roles [19]. The core mechanism of dry eye is initiated by the aqueous deficiency or evaporative state of the tear film, resulting in hyperosmolarity [2021]. Hyperosmolarity triggers mitogen-activated protein kinase or nuclear factor kappa beta, thereby promoting secretion of inflammatory cytokines such as interleukin 1 and tumor necrosis factor alpha that activate T-lymphocytes and matrix metalloproteinases [20]. These events increase damage to epithelial cells and goblet cells on the conjunctiva, decreasing gel-forming mucin secreted by goblet cells. The tear film then becomes unstable, increasing ocular surface damage and undesirable ocular symptoms [22]. Representative drugs targeting goblet cells of the conjunctiva include cyclosporine and diquafosol sodium.
This study confirmed the non-inferiority of cyclosporine nanoemulsion formulation compared to previously used cyclosporine emulsion formulation. Cyclosporine nanoemulsion formulation showed an equivalent effect on dry eye disease as diquafosol with a different mechanism of action. Both cyclosporine formulation groups, CN and CE, showed statistically significant improvement of the primary endpoint. Non-inferiority of the CN group was shown. Moreover, the CN group significantly decreased conjunctival staining score at week 12. This is because tear fluid including particles of the drug can remain relatively longer on the conjunctiva than the cornea. Moreover, in a study of cyclosporine nanoemulsion administered by oral gavage to Sprague Dawley rats, dosing with cyclosporine nanoemulsion showed higher serum drug levels than cyclosporine microemulsion. This is presumably due to smaller particle size facilitating absorption [23]. The CN group could have also absorbed drugs more easily in the conjunctiva. Problems with the emulsion formulation include ocular discomfort, blurred vision, burning/stinging, pain, non-homogeneity, and non-transparency, which were all significantly reduced with CN. However, stickiness sensation was significantly greater in the CN group. This is because the cyclosporine nanoemulsion formulation used in this trial contains xanthan gum as a mucoadhesive polymer and carboxymethylcellulose as a viscosity increasing agent.
In a comparison between the CN group and DQ group, TBUT and Schirmer test of the CN group showed consistent statistically significant improvements in all periods. However, in the DQ group, TBUT showed relatively late significant improvement at week 12 and the Schirmer test showed relatively short-term significant improvement at week 4.
In this study, participants had been diagnosed with dry eye disease. Their conjunctival goblet cells could have already been damaged to some degree due to reduced mucin secretion. Cyclosporine might have inhibited inflammation and apoptosis, thereby increasing goblet cell density and mucin secretion more efficiently and rapidly than diquafosol, which promotes mucin secretion in conjunctival goblet cells with some damage. In the Schirmer test, statistically significant improvements were shown at week 4 in both CN and DQ groups. However, such statistically significant improvement was no longer seen at week 12 in the DQ group. The effect of tear fluid secretion of conjunctival epithelial cells, which is promoted by diquafosol, might be inefficient in a long-term period due to epithelial cell damage by inflammation in dry eye disease. Although the exact mechanism is not yet fully understood, topical administration of cyclosporine accelerates tear secretion by releasing neurotransmitters from sensory nerves that interact with the parasympathetic component of the lacrimal functional unit [24]. The overall satisfaction of the CN group was superior to that of the DQ group. However, stickiness sensation was significantly greater in the CN group than in the DQ group. Instillation adherence in the CN group was also significantly superior to that in the DQ group because of fewer instillations.
In the safety assessment, no serious adverse event systemically related to using these three ophthalmic formulations was observed. Ocular adverse events such as ocular pain, irritation, foreign body sensation, and conjunctivitis were mild and easily treated. Because of its improved bioavailability, high blood concentration was a serious problem with cyclosporine emulsion; this concentration may lead to unintentional systemic adverse side effects. Highly sensitive high-performance liquid chromatography tandem-mass spectroscopy with a lower limit of quantitation of 0.1 ng/mL detected cyclosporine in 6 of 310 blood samples a fter topical a dministrations of 0.1% c yclosporine. Cyclosporine was not detected in patients administered with cyclosporine 0.05% [2526], and the concentrations of cyclosporine in plasma were extremely low. Thus, a systemic side effect was not anticipated. However, since this novel cyclosporine nanoemulsion has been demonstrated to improve bioavailability, further studies are needed to evaluate the safety of this novel drug by measuring its concentrations in plasma, tears, aqueous humor, and vitreous humor.
This trial has an unavoidable limitation of vehicle effect in that it was designed to compare three kinds of drugs with different dosing regimens. It would have been ideal to use a diquafosol vehicle in both cyclosporine groups for more accurate comparison. However, this was not practically available. Alternatively, hyaluronic acid was used 4 times daily. This may have caused another vehicle effect problem because hyaluronic acid could interfere with evaluation of drug efficacy. Despite this realistic limitation, 6 times daily dosing was considered more meaningful because it is more closely related to current practice in that dry eye patients usually use cyclosporine ophthalmic solutions in combination with lubricants.
This is a multicenter, randomized, and partially double-masked study involving control and minimization of selection bias and bias related to different numbers of instillations among groups. For the first time, this novel cyclosporine nanoemulsion formulation was evaluated and simultaneously compared to cyclosporine emulsion and diquafosol with different mechanisms of action. CN was equivalently efficient to CE and DQ and had significant improvements in several parameters for treatment of dry eyes.
Acknowledgements
This study was supported by an unrestricted educational grant from Taejoon Pharm (Seoul, Korea). The sponsor or funding organization had no role in the design or conduct of this research.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.