Association between Three Heavy Metals and Dry Eye Disease in Korean Adults: Results of the Korean National Health and Nutrition Examination Survey
Article information
Abstract
Purpose
To investigate the associations between blood heavy metal concentrations and dry eye disease using a Korean population-based survey.
Methods
This study included 23,376 participants >40 years of age who participated in the Korean National Health and Nutrition Examination Survey from 2010 to 2012. Blood concentrations of lead, cadmium, and mercury were measured in all participants. The associations between blood heavy metal concentrations and dry eye disease were assessed using multivariate logistic regression analyses.
Results
After adjusting for potential confounders, including age, sex, lifestyle behaviors and sociodemographic factors, the analyses revealed an increased odds ratio (OR) for dry eye disease with higher blood mercury concentrations (tertile 2: OR, 1.22; 95% confidence interval [CI], 0.91 to 1.64; tertile 3: OR, 1.39; 95% CI, 1.02 to 1.89; p = 0.039). The prevalence of dry eye disease was not associated with blood lead (tertile 2: OR, 1.15; 95% CI, 0.87 to 1.51; tertile 3: OR, 0.83; 95% CI, 0.59 to 1.16; p = 0.283) or cadmium (tertile 2: OR, 1.05; 95% CI, 0.77 to 1.44; tertile 3: OR, 1.15; 95% CI, 0.84 to 1.58; p = 0.389) concentrations. There were no significant associations between any of the three heavy metals and dry eye disease in males after adjusting for potential confounding factors, but blood mercury concentrations in females were associated with dry eye disease (tertile 2: OR, 1.18; 95% CI, 0.83 to 1.69; tertile 3: OR, 1.58; 95% CI, 1.12 to 2.24; p = 0.009).
Conclusions
Mercury concentrations in blood were associated with dry eye disease. Our results suggested that controlling environmental exposure to mercury may be necessary to reduce the incidence of dry eye disease.
Dry eye disease is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film. Affected patients typically present with tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities [1]. There are various risk factors for dry eye disease including older age, female sex, lifestyle factors, lower socioeconomic status, and previous ocular surgery (including cataract and refractive surgeries) [1]. However, because of the multiple mechanisms involved in the pathogenesis of dry eye disease, many additional risk factors likely remain to be elucidated.
Heavy metals are metallic elements that have a relatively high density compared to water [2]. Sources of heavy metals in the environment include geogenic, industrial, agricultural, pharmaceutical, and atmospheric sources, as well as domestic effluents [3]. As a result of the increased use of heavy metals in industrial, agricultural, domestic, and technological applications, the likelihood of being exposed to t hese materials has risen dramatically [4]. A mong the many types of heavy metals, arsenic, cadmium, chromium, lead, and mercury have t he greatest toxicity. These heavy metals are known to induce damage to multiple organs, even at low levels of exposure [5]. Several studies addressing the associations between heavy metals and ocular diseases have been published, but there have been few investigations of the association between dry eye disease and heavy metal exposure [67]. It is therefore necessary to clarify the associations between heavy metals and dry eye disease. We investigated possible associations between three toxic heavy metals—lead, cadmium, and mercury—and dry eye disease using a large, nationally representative population-based cohort for the Republic of Korea. Additionally, because the prevalence of dry eye disease differs by sex, we also examined the associations between heavy metal exposure and dry eye disease according to sex.
Materials and Methods
Study population
This study was conducted using data from a cross-sectional analysis of the fifth (2010–2012) Korean National Health and Nutrition Examination Survey (KNHANES) completed by the Korea Centers for Disease Control and Prevention. The KNHANES provides data on national health insurance status, health behaviors, and nutritional status for the non-institutionalized civilian population of the Republic of Korea using multistage, stratified, probability-clustered sampling methods [89].
Among 23,376 participants from the 2010–2012 KNHANES who underwent an ophthalmic examination, we excluded those aged below 40 years, and those for whom dry eye symptoms, heavy metal exposure, and/or eye surgery history data were missing. The final study population comprised 2,811 participants. This study was approved by the institutional review board of the Soonchunhyang University Bucheon Hospital (2018-06-021) and written informed consent was obtained from all the KNHANES participants by the Korea Centers for Disease Control and Prevention and all data are publicly available.
Heavy metal concentrations
Blood concentrations of lead and cadmium were measured using graphite furnace atomic absorption spectrometry (AAnalyst 600; Perkin-Elmer, Turku, Finland), and mercury concentrations were measured using the gold amalgamation method (DMA-80; Milestone, Sorisole, Italy). Blood samples were collected and stored in trace element ethylenediaminetetraacetic acid tubes (Becton Dickinson, Franklin Lakes, NJ, USA) for heavy metal assays. The detection limits were 0.223, 0.087, and 0.05 µg/L for lead, cadmium, and mercury, respectively. The concentrations of all samples were greater than the detection limits. All blood analyses were carried out by the Seoul Medical Science Institute, which is certified by the Korean Ministry of Health and Welfare. Internal and external quality control procedures for this institute were described in a previous study [7].
Definition of dry eye symptoms
Since July 2008, the Korean Ophthalmological Society has participated in the KNHANES by performing ophthalmic interviews and examinations of study participants. Participants were considered to have dry eye disease if they responded positively to questions addressing symptoms such as persistent dryness or eye irritation. If the participant stated that they only had these symptoms “sometimes” or “occasionally”, they were classified as not having dry eye disease.
Other variables
Demographic variables included age, sex, area of residence, occupation, income, and education level. These were obtained from health interviews. Age was classified as 40 to 49, 50 to 59, or ≥60 years. Education status was divided into two groups: middle school or lower, and high school or higher. Family income was split into upper and lower 50% groups. The other covariates were health-related behaviors (smoking status, alcohol consumption, and body mass index [BMI]), history of ophthalmic surgery, and family history of ophthalmic disease. Smoking status was categorized as non-smoker, ex-smoker, or current smoker. Alcohol consumption was categorized as none, occasional (<2 drinks/wk) or frequent (≥2 drinks/wk). BMI was classified as <18.5, 18.5 to 24.9, or ≥25 kg/m2.
Statistical analyses
Given the complex sampling design, all statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC, USA), and the results yielded nationally representative estimates of the prevalence of dry eye disease. The PROC SURVEY procedure was used for all analyses. To investigate the prevalence of dry eye disease according to participant characteristics, the Rao-Scott chi-square test was used. Blood concentrations of lead, mercury, and cadmium were log-transformed, and then geometric means and proportional changes were compared by analysis of covariance after adjusting for age group, sex, region of residence, occupation, education level, smoking status, drinking status, family income, family history of ophthalmic disease, and history of ophthalmic surgery. Multivariate logistic regression analyses were conducted to assess the associations between dry eye disease and blood heavy metal concentrations. For the logistic regression analyses, Four models were established: model 1 used simple logistic regression; model 2 adjusted for age group and sex; model 3 adjusted for model 2 and lifestyle behaviors (smoking status, drinking status, and region of residence); and model 4 adjusted for model 3 and sociodemographic factors (educational level, occupation, family income, family history of ophthalmic disease, and history of ophthalmic surgery). Odds ratios (ORs) and 95% confidence intervals (CIs) for the prevalence of dry eye disease were determined. All p-values of less than 0.05 were considered to indicate statistical significance.
Results
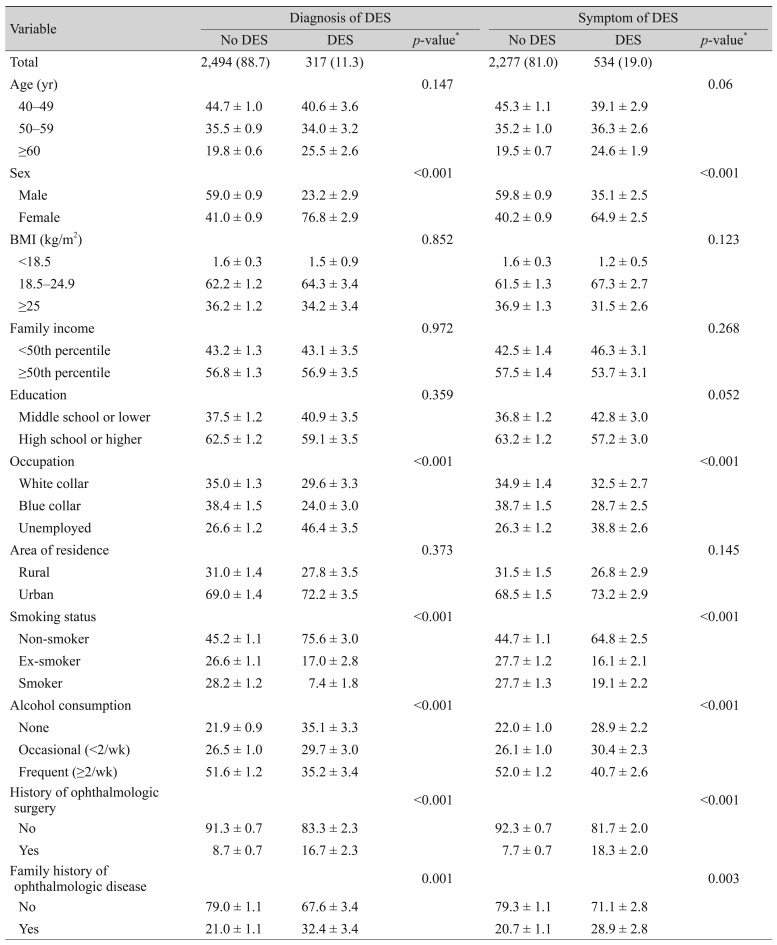
Table 1 presents the associations between the prevalence of dry eye disease and the demographic characteristics of our study population. Of the 2,811 participants, 534 had symptoms of dry eye disease and 2,277 had no symptoms, while 317 participants were diagnosed with dry eye disease and 2,494 were not. Signs and symptoms of dry eye disease were significantly more prevalent in females than in males (p < 0.001), while family income, educational level, and area of residence did not differ according to the presence of dry eye disease. Regarding occupation, unemployed participants were more likely to suffer from dry eye disease than white or blue collar workers (p < 0.001). Contrary to the findings of many other reports, the prevalence of dry eye disease was greater in non-smokers than in smokers or ex-smokers (p < 0.001), in non-drinkers than alcohol drinkers (p < 0.001), and in participants with a history of ophthalmic surgery (p < 0.001) or family history of ophthalmic disease (p = 0.001).
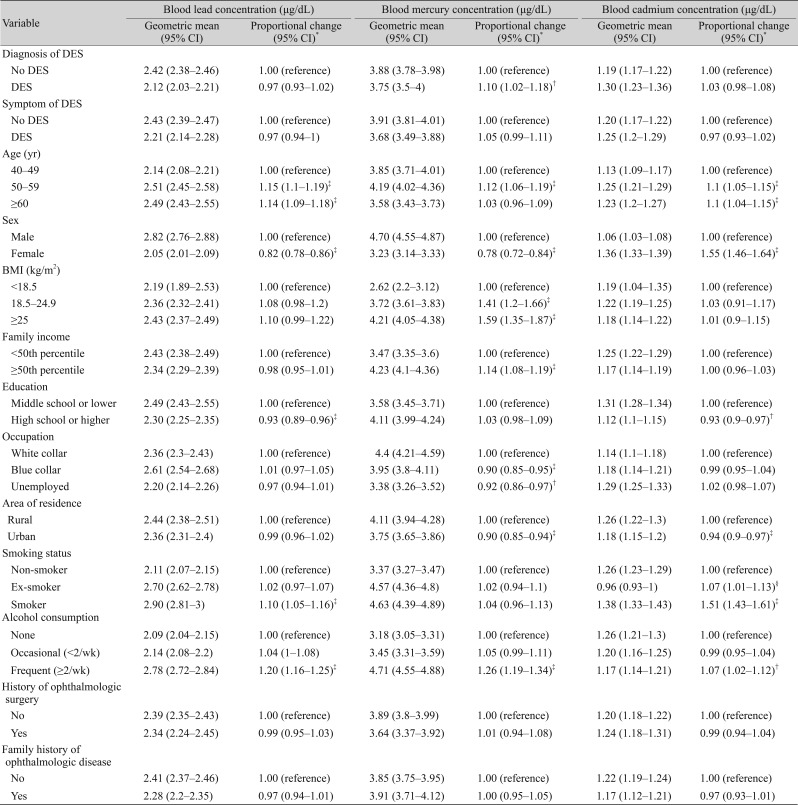
Table 2 presents the associations between blood concentrations of heavy metals and demographic factors. Blood lead concentrations were greater in the older, smoking and frequent alcohol consumption groups compared to the younger, non-smoking and non-drinking groups, respectively (p < 0.001). Blood lead concentrations were lower in more educated participants and in females. Blood mercury concentrations were greater in the high BMI group, and in the dry eye disease, high family income, and frequent alcohol consumption groups (p < 0.001), and were lower in females, blue collar or unemployed workers, and those living in urban areas (p < 0.001). Blood cadmium concentrations were significantly greater in the 50- to 59-year-old age group compared to the <50 age group, and in females versus males (p < 0.001). In addition, the cadmium blood concentration was greater in smokers, those with a lower education level, and those living in rural areas (p < 0.001).
Table 3 presents the associations between blood heavy metal concentrations and dry eye disease. Multivariate logistic regression analyses were used to calculate ORs by dividing heavy metal concentrations into tertiles. After adjusting for age and sex, lifestyle behaviors (smoking status, alcohol consumption, and region of residence), and sociodemographic factors (education level, occupation, family income, family history of ophthalmic disease, and history of ophthalmic surgery), the prevalence of dry eye disease was not significantly associated with blood lead (tertile 2: OR, 1.15; 95% CI, 0.87 to 1.51; tertile 3: OR, 0.83; 95% CI, 0.59 to 1.16; p = 0.283) or cadmium (tertile 2: OR, 1.05; 95% CI, 0.77 to 1.44; tertile 3: OR, 1.15; 95% CI, 0.84 to 1.58; p = 0.389) concentrations. Furthermore, blood mercury concentrations were directly proportional to the prevalence of dry eye disease (tertile 2: OR, 1.22; 95% CI, 0.91 to 1.64; tertile 3: OR, 1.39; 95% CI, 1.02 to 1.89; p = 0.039).
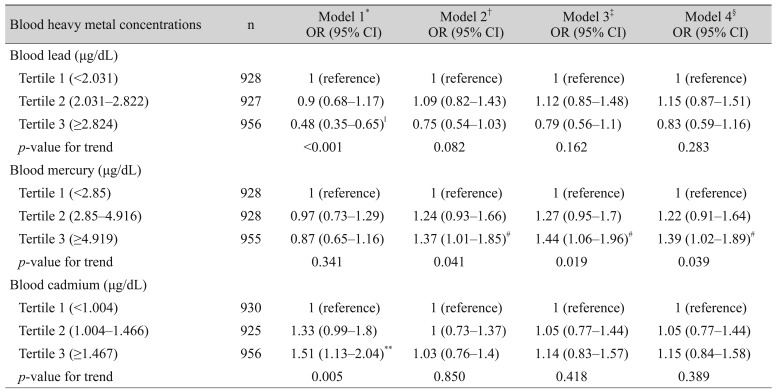
OR (95% CI) for diagnosis of dry eye syndrome according to the blood heavy metal concentrations in Korean adults over 40-aged
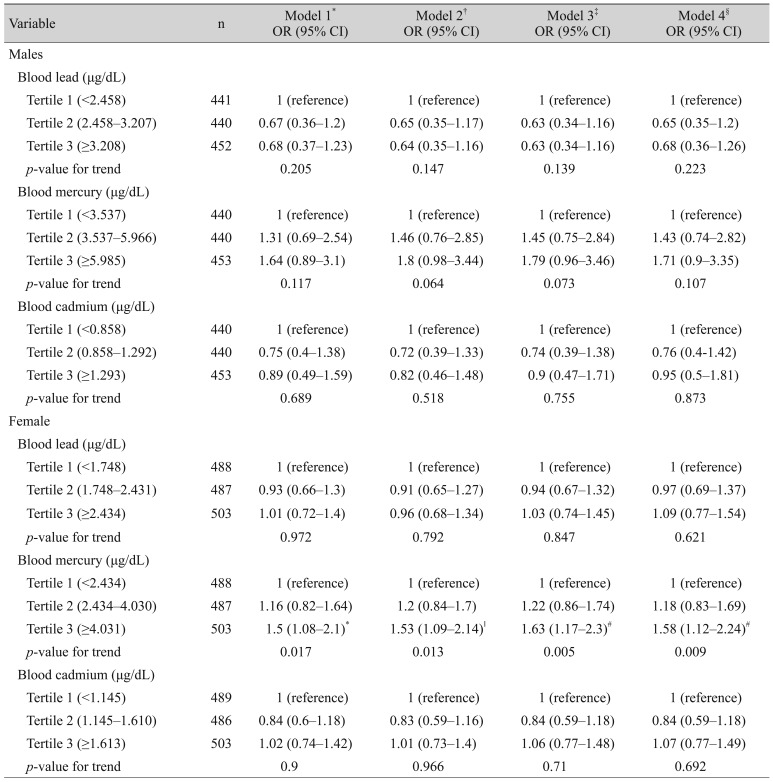
Table 4 presents the associations between blood heavy metal concentrations and dry eye disease according to sex. None of the three heavy metals were associated with dry eye disease in males after adjusting for potential confounding factors. In females, there was a positive association between the blood mercury levels and dry eye disease in adjusted model 4 (male; tertile 2: OR, 1.43; 95% CI, 0.74 to 2.82; tertile 3: OR, 1.71; 95% CI, 0.9 to 3.35; p = 0.107; female; tertile 2: OR, 1.18; 95% CI, 0.83 to 1.69; tertile 3: OR, 1.58; 95% CI, 1.12 to 2.24; p = 0.009).
Discussion
We investigated the associations between dry eye disease and heavy metal concentrations in blood using a large, nationally representative population-based database for the Republic of Korea. There were no associations between blood lead or cadmium concentrations and dry eye disease. Mercury was the only heavy metal found to be significantly associated with dry eye disease, and subgroup analyses revealed that this association only applied to females.
Mercury is a ubiquitous environmental toxicant and pollutant that induces severe changes in body tissues and causes numerous adverse health effects [10]. In animal studies, ionic mercury has been found in the vitreous humor, and is transported to the retinal pigment epithelium where it accumulates in the plexiform layer and ganglion cells [111213]. Reduced color vision and visual acuity have been reported in mercury-exposed populations [14]. The duration of exposure and amount of methylmercury ingested may influence the severity of the visual disturbance [15]. In a previous study investigating the relationships between mercury and dry eye symptoms based on the KNHANES data, Chung and Myong [7] noted an association between dry eye symptoms and blood mercury concentrations. These findings are consistent with the results of our study, with both studies showing a positive association between blood levels of mercury and dry eye disease in the Korean population; however, in our study we analyzed the associations between blood concentrations of toxic heavy metals and actual diagnoses of dry eye disease rather than dry eye symptoms, which yielded more accurate results.
There are several hypothetical mechanisms by which mercury may cause dry eye symptoms. The first hypothesis is that neurotoxicity induced by methylmercury exposure may affect the lacrimal glands. The main lacrimal glands are innervated by both parasympathetic and sympathetic nerves [1617]. Stimulation of the parasympathetic and sympathetic nerves induces release of neurotransmitters that activate secretion of water and electrolytes from the lacrimal gland [18]. The possibility that neurotoxicity secondary to mercury exposure may influence the development of dry eye disease was first identified in a study that showed mercury vapor may damage the sympathetic nervous system. We speculate that if the autonomic nervous system is damaged by mercury, secretions from the glands may decrease, resulting in dry eye disease [19]. The second hypothetical mechanism is that acceleration of free radical reactions as a result of mercury exposure may cause dry eye disease. This theory may be supported by a previous study that showed that methylmercury exposure induces conjunctival inflammation through increased formation of reactive radicals and acceleration of free radical reactions [2021]. The inflammation at the ocular surface promotes epithelial disease and eventually results in dry eye symptoms [22]. Finally, tear film hyperosmolarity may explain the association between blood mercury concentrations and dry eye disease. Tear film hyperosmolarity has been identified as one of the main mechanisms underlying dry eye disease. A positive association between plasma osmolarity and tear osmolarity has been reported, and both are increased in patients with dry eye disease or systemic dehydration [232425]. Given previous animal studies have shown that mercury toxicity may increase serum osmolarity, increased blood mercury concentration may alter serum osmolarity, leading to increased tear hyperosmolarity and dry eye disease [262728].
Previous studies showed that cadmium and lead toxicity can cause various ocular conditions. These two toxic heavy metals have been found in all of the pigmented ocular tissues, and accumulate in other ocular tissues, especially in the retinal pigment epithelium and choroid [29], where they could induce oxidative stress by producing reactive oxygen species [30]. Because oxidative stress is thought to play an important role in age-related macular degeneration [31], cadmium and lead ions have also been implicated in this disorder [32]. In another report, blood cadmium concentrations were positively associated with the prevalence of open-angle glaucoma in teenagers [6]. Although our study did not find any positive associations between dry eye disease and blood cadmium or lead concentrations, possible associations between oxidative stress caused by excessive free radicals from toxic heavy metals (including lead and cadmium) and dry eye disease have been suggested previously [33]. The potential ocular toxicity of these heavy metals therefore requires further study.
We also investigated the associations between dry eye disease and blood heavy metal concentrations in male and female participants in our study. In this subgroup analysis, we found a positive association only in females. The reason for this result is unclear, but one possible hypothesis involves differences in the distribution and excretion of mercury according to sex [3435]. Additionally, the greater prevalence of dry eye disease among females may also have contributed to our results.
This study had several limitations. First, we did not define dry eye disease according to the results from physical examination or dry eye tests. Due to the inherent limitations of the data provided by the Korea Centers for Disease Control and Prevention, we used a questionnaire to define dry eye disease. Further studies using more strict diagnostic criteria for dry eye disease are needed to reveal the true associations between heavy metal concentrations in blood and dry eye disease. Second, we could not determine temporal a ssociations o r c ausality because of the cross-sectional nature of the study. Longitudinal studies are necessary to confirm our results. Third, other unmeasured factors could have confounded the reported associations. Fourth, there are heavy metals that we did not investigate, including arsenic, magnesium, and zinc. Finally, the odds ratios for diagnosis of dry eye disease according to the level of blood mercury concentration were only marginally elevated; however, since environmental variables are becoming important risk factors for dry eye disease [36], the results of our study may have important clinical implications with respect to the pathogenesis of dry eye disease by demonstrating a possible association between increase blood mercury concentrations and dry eye disease. This study also had several important strengths. First, it included a large number of participants, which may have minimized any selection bias; furthermore, the large population-based sample was representative of the population of the Republic of Korea. Second, we considered and adjusted for various potential confounders that may have affected the true associations between blood heavy metal concentrations and dry eye disease, including sex, age, area of residence, education level, household income, smoking and alcohol consumption.
In this study, we investigated the associations between blood heavy metal concentrations and dry eye disease using a representative national sample of Korean adults. The results showed that blood concentrations of mercury were associated with dry eye disease, especially in females. Although further experimental and longitudinal studies are needed to confirm the causal association between blood mercury concentrations and dry eye disease, our results suggest that controlling environmental exposure to mercury may be necessary to address the increasing incidence of dry eye disease.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D-1A1B03029944).
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.