High-grade Mucoepidermoid Carcinoma of the Lacrimal Gland
Article information
Dear Editor,
Mucoepidermoid carcinoma is a very rare malignancy of the lacrimal gland [1]. To the best of our knowledge, only one case of intermediate-grade mucoepidermoid carcinoma of the lacrimal gland has previously been reported in Korea [2]. We present the first report of a high-grade mucoepidermoid carcinoma of the lacrimal gland.
A 79-year-old woman presented with progressive ptosis and swelling of the left eyelid for five months (Fig. 1A). Her best-corrected visual acuity was 20 / 50 in the left eye, with a relative afferent pupillary defect. There was also hypoglobus of the left eye and severely limited upward movement. Exophthalmometry demonstrated a 6-mm proptosis of the left eye. An orbital computed tomography scan showed a heterogeneous, poorly defined mass occupying the superior orbital area. The mass displaced the left globe inferiorly and caused bony destruction of the lateral wall (Fig. 1B). A gadolinium-enhanced, fat-suppressed magnetic resonance image showed the orbital mass with heterogeneous enhancement, penetrating the lateral orbital wall and spreading to the temporalis muscle (Fig. 1C).
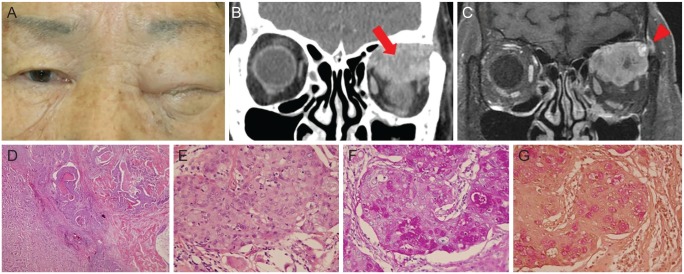
(A) A 79-year-old woman presented with complete ptosis and swelling of the left eyelid. Informed consent was received from the patient. (B) Preoperative coronal view of orbital computed tomography image showed a heterogeneous, poorly defined mass occupying the left superior orbit (arrow). (C) A gadolinium-enhanced, fat-suppressed magnetic resonance image showed the orbital mass with heterogeneous enhancement, penetrating the lateral orbital wall and spreading to the temporalis muscle (arrowhead). (D,E) Hematoxylin and eosin staining showed infiltrating tumor cells consisted of squamous epithelioid and clear mucinous cells (×40) and some tumor nests were comprised of mucous goblet cells and intermediate cells with a central lumen containing mucin (×400). The presence of intracellular mucin was confirmed with positive (F) periodic acid-Schiff and (G) mucicarmine staining (×400).
An incisional biopsy revealed an intermediate to high-grade mucoepidermoid carcinoma. Ultrasonography demonstrated an intake orbital roof. Systemic evaluations did not reveal signs of metastasis. Lid-sparing exenteration with lateral orbitectomy was then performed. A split-thickness skin graft was harvested from the right thigh and placed into the left eye socket. A histopathological examination revealed infiltrating tumor cells consisting of squamous epithelioid and clear mucinous cells. Some tumor nests were comprised of mucous goblet cells and intermediate cells, with a central lumen containing mucin. The presence of intracellular mucin was confirmed with positive periodic acid-Schiff and mucicarmine staining (Fig. 1D-1G). Approximately 30% of the mass involved an intracystic component, with five mitoses per 10 high power field images. There was also necrosis in this area, without neural invasion. A diagnosis of high-grade mucoepidermoid carcinoma (T4cN0M0) was made according to the World Health Organization/Armed Forces Institute of Pathology grading system and the 8th American Joint Committee on Cancer staging system.
The patient received 6,000 cGy of radiation over 6 weeks, and was tumor-free for 13 months. Unfortunately, after 13 months, she developed parotid metastasis. She declined radical neck dissection, parotidectomy, and radiation. She survived for another 14 months.
Histopathological findings of mucoepidermoid carcinomas involve mucus-secreting, epidermoid, and intermediate cells [1]. There is a standard histological grading system for mucoepidermoid carcinomas of the salivary gland. These tumors are divided into low-grade, intermediate-grade, and high-grade classifications based on their cellular features, including necrosis, mitotic figures, and atypical nuclei. Low-grade tumors have abundant cytoplasm with large, well-differentiated cells, a relative paucity of epidermoid cells, small nuclei, and no mitotic figures. High-grade tumors are characterized by increased cellularity, smaller cells with an abnormal nuclear-to-cytoplasmic ratio, hyperchromatism, and frequent mitotic figures.
Similar to salivary gland carcinomas, the management of mucoepidermoid carcinomas of the lacrimal gland depends on the histological grade [1345]. Low-grade tumors are treated with extirpation with or without adjuvant radiation. High-grade tumors need exenteration, as well as radiation, and resection of the involved orbital bone.
Thorvaldsson et al. [3] described that the prognosis of mucoepidermoid carcinomas of the major salivary gland also depends on the histological grade. With a median follow-up of 12 years, the group found survival rates of 100% and 97% for patients with grade 1 and 2 tumors, respectively. In contrast, those with grade 3 tumors had only 43% survival over the 12 years. When comparing all types of lacrimal and salivary gland tumors, carcinomas with similar histologies have worse prognoses when they occur in the lacrimal gland versus the salivary gland. High-grade mucoepidermoid carcinomas of the lacrimal gland can metastasize to the lung, brain, and mediastinum. Only two cases have been reported in which patients with high-grade mucoepidermoid carcinomas with orbital exenteration and adjuvant radiation have survived.
In conclusion, although rare, high-grade mucoepidermoid carcinomas of the lacrimal gland have poor survival rates. Given their poor prognosis, these tumors require radical surgery and radiation treatment with close follow-up.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.