Optical Coherence Tomography Measurement and Visual Outcome in Acute Central Retinal Artery Occlusion
Article information
Abstract
Purpose
This study investigated visual acuity (VA) values and differences depending on optical coherence tomography (OCT) findings in patients with acute central retinal artery occlusion (CRAO).
Methods
A retrospective chart review was performed on patients with acute CRAO who underwent macular and disc OCT. We evaluated changes in macular thickness and retinal nerve fiber layer (RNFL) thickness after acute CRAO onset based on OCT. We also determined the association of thickness changes with VA improvement.
Results
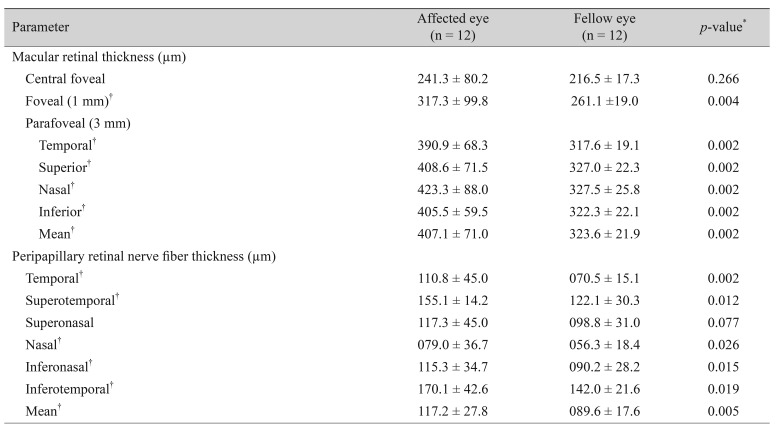
This study involved both eyes in a total of 12 patients with acute CRAO. A significant increase was observed in foveal (1 mm) thickness (p = 0.002), parafoveal (3 mm) thickness (p = 0.002), and peripapillary RNFL thickness (p = 0.005) in affected eyes with CRAO, but not in central foveal thickness (p = 0.266). A significant small difference in both eyes (affected eye – fellow eye) was shown in foveal (1 mm) and mean parafoveal (3 mm) thickness in the improved VA group (p = 0.008 and p = 0.004, respectively), but not in central foveal or peripapillary RNFL thickness (both p = 0.283).
Conclusions
Both macular and RNFL thickness increased in patients with acute CRAO. RNFL thickness decreased over time with progression of RNFL atrophy. Less macular damage caused by acute CRAO could be predicted by a small difference in macular thickness between eyes (affected eye – fellow eye). In such cases, patients had a greater chance of VA improvement.
Central retinal artery occlusion (CRAO), first described by von Graefes in 1859, is an ocular emergency that usually occurs with sudden vision loss in one eye [1]. Retinal artery occlusion (RAO) originates by the same pathophysiological mechanism as stroke caused by thromboembolism and has been found to increase the risk of stroke by 1.78 times in all Korean adults and 3.11 times in adults aged 65 years and older according to a nationwide cohort study [23]. RAO is significantly associated with hypertension, ischemic heart disease, atrial fibrillation, diabetes mellitus, chronic renal failure, hyperlipidemia, and other conditions. These conditions are also linked to an increased risk of stroke [3].
Optical coherence tomography (OCT) is a noninvasive imaging technique that can provide in vivo cross-sectional images of retinal microstructures. In recent years, many studies have examined patients with RAO using OCT [45678]. Schmidt et al. [9] categorized CRAO as incomplete, subtotal, or total CRAO based on the degree of vision loss, retinal edema, and arterial blood flow delay. They demonstrated that final visual prognostic outcome depends on the stage classified. Ahn et al. [4] showed that retinal and choroidal structural changes predominantly differed depending on the stage of CRAO determined using OCT. They showed that OCT can be a useful imaging biomarker for diagnosis, staging, and prognosis of CRAO.
However, no quantitative analysis has been done to explore CRAO with peripapillary retinal nerve fiber layer (RNFL) thickness, though one study identified disc edema in 22% of patients with permanent CRAO [10]. For these reasons, we quantitatively analyzed macular thickness and peripapillary RNFL thickness in affected and fellow eyes using OCT in CRAO patients. We also compared changes in macular thickness and RNFL thickness of both eyes between two groups with and without improvement in visual acuity (VA).
Materials and Methods
This study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Severance Hospital of Yonsei University (4-2016-0384). Due to the retrospective nature, informed consent was waived.
Study subjects
The patient database in Severance Hospital of Yonsei University was searched, and patient records between March 2010 and February 2016 were reviewed. Patients included were those who met the following criteria: 1) diagnosed with acute CRAO without cilioretinal artery sparing, 2) presented within seven days of decrease in visual acuity, 3) spectral dominant optical coherent tomography (SD-OCT) examination performed within 6 days, and 4) underwent assessment of macular thickness and peripapillary RNFL thickness. Exclusion criteria were 1) the presence of ocular diseases such as macular degeneration, 2) history of retinal artery occlusion or stroke, 3) suspicion of iatrogenic CRAO, 4) followed up for less than one month, or 5) management with other therapies except conservative treatment.
All patients received conservative treatment, including ocular massage, anterior chamber paracentesis, and intraocular pressure-lowering agents. No patients underwent intra-arterial or intravenous thrombolysis.
Ophthalmic examinations
All patients underwent comprehensive ophthalmic examinations including best-corrected VA, noncontact tonometry, slit-lamp biomicroscopy, indirect ophthalmoscope, fundus photography, fundus fluorescein angiography, and SD-OCT examination. Fundus angiography was carried out in both eyes (Fig. 1A). SD-OCT examination was performed using Spectralis (Heidelberg Engineering, Heidelberg, Germany). SD-OCT was carried out in macular and peripapillary RNFL mode in both eyes. When examining the macular scan, a range of 20 by 20 degrees of macular image was obtained using the Volume Scan method with the Spectralis OCT machine. On macular scan, a thickness map was developed showing an average thickness of 1-mm-, 3-mm-, and 6-mm-diameter zones from the fovea by measuring the distance from RNFL to the basement membrane (Fig. 1B, 1C). On peripapillary RNFL scan, RNFL thickness was measured within a 3-mm-diameter circle of the disc center (Fig. 1D, 1E). Two authors (HKK and JYY) manually corrected the center of the fovea and baseline RNFL to prevent segmentation errors caused by edema or thickening of inner and outer retinal layers. We used the average thicknesses of the 1-mm zone (define as foveal thickness) and the 3-mm zone (defined as parafoveal thickness). The average thickness of the 6-mm zone was excluded due to missing data when adjusting for the macular center. These measurements might be influenced by individual variations such as age and health status. For this reason, the difference in measurements between affected and fellow eyes was used as a variable to compare patients. Assuming macular thickness and RNFL thickness were the same in both eyes, differences in measurements of the two eyes were defined as macular thickness change and peripapillary RNFL thickness change.

Fundus angiography and optical coherence tomography of central retinal artery occlusion patient at initial visit (patient 1). Fundus angiography and optical coherence tomography scans taken at initial visit in patient 1 with central retinal artery occlusion. (A) shows disc leakage in the affected eye (the right eye) 10 minutes after fundus angiography. (B) shows macular thickness, and (C) represents the graph of measurements, indicating an increase of macular thickness on the affected side. (D) shows scan images of peripapillary retinal nerve fiber layer thickness, and (E) represents the graph of thickness values, revealing a thick retinal nerve fiber layer thickness in the affected eye compared with the fellow eye.
Statistical analysis
Data for continuous variables were expressed as mean ± standard deviation. The Wilcoxon signed-rank test was used to assess changes in macular thickness and RNFL thickness in affected and fellow eyes. The Mann-Whitney U-test was used to compare differences in macular thickness and RNFL thickness changes between improved VA and non-improved VA groups. Spearman's correlation was used to identify the correlation between foveal or parafoveal thickness and RNFL thickness. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA).
Results
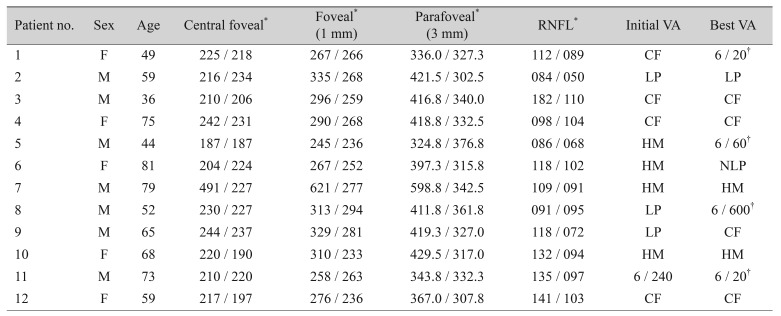
This study involved 12 affected and 12 fellow eyes in a total of 12 patients. The subjects were 7 men and 5 women, and their mean age was 61.7 ± 14.4 years (range, 36 to 81 years). Their initial VA was very poor (count fingers to light perception), and improvement in VA from baseline to higher than count fingers was seen in 4 patients (Table 1).
According to comparison of macular thickness and RNFL thickness values measured with OCT, foveal thickness increased in the affected eye (p = 0.002), and parafoveal thickness increased at all temporal, superior, nasal, and inferior areas (all p < 0.005), but central foveal thickness did not (p = 0.266). Meanwhile, RNFL thickness increased significantly in the affected eye in all areas except the superonasal region (Table 2).
Differences in macular and RNFL thickness, defined as the difference between the affected and fellow eyes of each patient (OCT measure of affected eye – OCT measure of fellow eye), were compared between the improved VA group and the non-improved VA group. When we defined the improved VA group as those with a final VA greater than 6 / 600, differences in foveal and parafoveal thickness were significantly smaller in the improved VA group (p = 0.008, p = 0.004, respectively). However, no statistical difference was found in the difference in parapapillary RNFL thickness measurements between the improved VA and non-improved VA groups (Table 3). Even if we defined the improved VA group as those with a final VA greater than 6 / 60, differences in foveal and parafoveal thickness were significantly smaller in the improved VA group (both p = 0.009, data not shown), although there was no statistical difference in parapapillary RNFL thickness (p = 0.283, data not shown).

Differences in macular thickness and peripapillary RNFL thickness change of affected and fellow eyes
We additionally evaluated the associations between baseline OCT features and final VA. When we evaluated the association between final VA and baseline OCT feature differences in the two eyes, final VA showed a significant correlation with baseline foveal and parafoveal thickness differences (p = 0.011, p = 0.001, respectively), but no significant correlation with peripapillary RNFL thickness difference (p = 0.846). When we evaluated the associations between final VA and baseline OCT features in affected eyes, final VA showed a significant correlation with baseline foveal and parafoveal thickness (p = 0.071, p = 0.011, respectively), but no significant correlation with peripapillary RNFL thickness (p = 0.842) (Table 4).
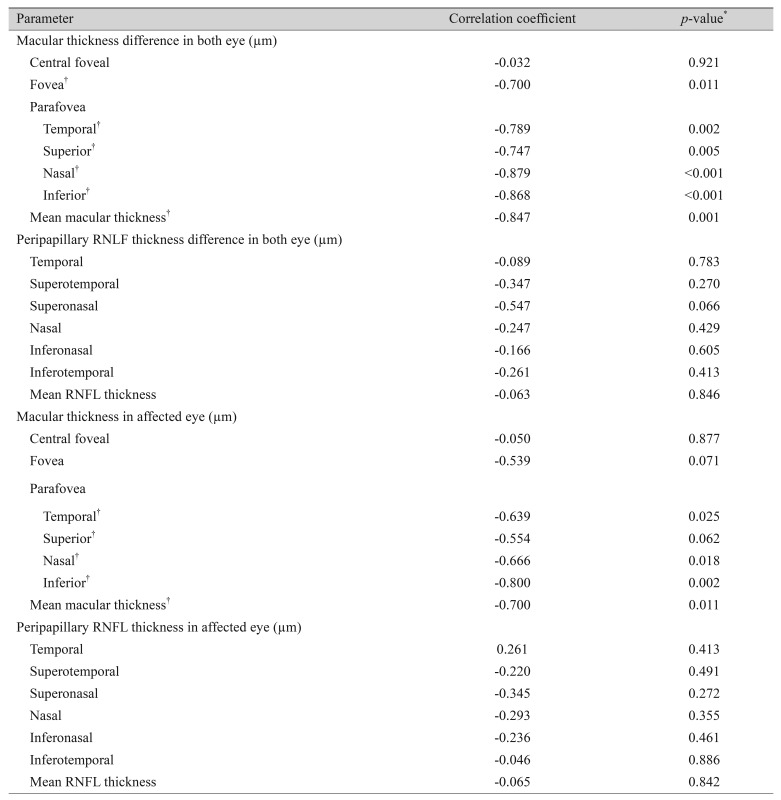
Association between baseline optical coherence tomography measurements and final best visual acuity in patients with acute central retinal artery occlusion
We did not find correlations between foveal or parafoveal thickness and RNFL thickness (p = 0.951, p = 0.538, data not shown) or between foveal or parafoveal thickness change and RNFL thickness change (p = 0.624, p = 0.806, data not shown).
Discussion
Acute retinal arterial ischemic disorders are mainly classified as CRAO, branched retinal artery occlusion, or cotton wool spots and amaurosis fugax [11]. The rate of spontaneous visual improvement in CRAO patients is very low. Spontaneous recanalization can occur within 48 to 72 hours, but it is partial and has little effect on visual improvement [12]. Treatment options available for CRAO are as follows according to mechanism: 1) increasing blood oxygen, 2) reduction of intraocular pressure and hence increase of retinal artery perfusion, 3) reduction of retinal edema, 4) lysing or dislodging the clot, and 5) thrombolysis of the embolus [13]. The use of thrombolysis of the embolus with recombinant tissue plasminogen activator remains controversial in patients with CRAO. Some studies have reported the benefits of intra-arterial or intravenous thrombolysis [141516], while other studies have found no benefits [1718]. This study involved patients who received conservative treatment only via an increase in retinal artery perfusion by reducing intraocular pressure.
Before the use of OCT, fundus photography was performed as a screening tool in patients with CRAO. According to Hayreh and Zimmerman [10], the main findings for permanent CRAO were cherry-red spot (90%), retinal opacity (58%), box-carrying (19%), retinal arterial attenuation (32%), optic disc edema (22%), and pale disc (39%). Since SD-OCT has been widely used in clinical imaging, studies have predominantly explored CRAO based on micro-sectional images. These studies have shown that CRAO can lead to macular edema caused by thickening of inner and outer retinal layers due to ischemia of the superficial and deep capillary plexus [7]. The increase of macular thickness in the initial phase of ischemia can lead to macular thickness decrease over time as atrophic change occurs [68]. Microvascular ischemia appears to affect the entire retina, not only the macular region. Subsequently, disc edema is accompanied by RNFL thickening, and thickness decrease is thought to develop following RNFL atrophy. When we examined changes in RNFL thickness in a patient over time, RNFL thinning progressed rapidly after CRAO onset (Fig. 2A–2F).

Changes in retinal nerve fiber layer (RNFL) thickness in the affected eye with central retinal artery occlusion (CRAO) (patient 4). Optical coherence tomography scans taken over time in Patient 4 with CRAO. (A), (B), and (C) are peripapillary cross-sectional images and (D), (E), and (F) are schematic graphs of RNFL thickness. (A) and (D) are images taken at the initial visit (1 day after CRAO attack), (B) and (E) are images taken 2 months after onset, and (C) and (F) are peripapillary RNFL optical coherence tomography images and thickness assessed 6 months after onset, revealing a reduction in RNFL thickness over time after CRAO attack.
Several studies have been performed to determine the association of macular thickness measured with SD-OCT with VA improvement. Multiple studies have suggested the association of initial macular thickness with improvement in VA [4519]. On the contrary, one study observed no relationship between them [6]. These contradicting results are attributed to selection bias. The incidence of retinal artery occlusion is about 0.85 cases per 100,000 persons per year [20], and CRAO is even more rare. It is difficult to adjust for confounding variables affecting macular thickness, including patient age and distribution of stage, in all patients with CRAO. Therefore, this controversy might have arisen from different research groups. Therefore, we conducted a comparative analysis considering the difference in macular thickness between affected and fellow eyes as a novel variable defined as macular thickness change. Although a significantly smaller change in macular thickness was observed in the improved VA group, the results from this research cannot be generalized due to a relatively small sample size.
No correlation was found between macular thickness (foveal or parafoveal thickness) and RNFL thickness in this study. This outcome might be attributable to different anatomical locations of ischemic damage and the different types of blood vessels involved. Since blood supply to the optic disc arrives via retinal and posterior ciliary arteries [21], optic disc ischemia can be less severe as the posterior ciliary arteries play a compensatory role for ischemia in CRAO patients.
This study has several limitations. First, we conducted research on a small number of patients. Therefore, although actual RNFL changes can cause significant differences in visual outcome, there is a possibility that the results are not significant in this study. Second, morphological changes of macular and RNFL thickness might be stage-dependent and influenced by symptom duration. We did not consider these factors.
In conclusion, macular thickness measured by SD-OCT in the affected eye increased significantly compared with the fellow eye in patients with acute CRAO. A significant increase was also found in RNFL thickness, which then decreased over time with atrophic change in patients with acute CRAO. We demonstrated that a smaller difference in macular thickness between affected and fellow eyes can reveal less severe macular damage caused by acute CRAO. A higher probability of VA improvement is anticipated in patients with less severe macular damage. Therefore, macular thickness difference between affected and fellow eyes evaluated using SD-OCT in patients with acute CRAO can predict VA improvement.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.