The Pathologic Characteristics of Pingueculae on Autofluorescence Images
Article information
Abstract
Purpose
To analyze the autofluorescence (AF) properties of pinguecula using cobalt-blue and yellow filters and to investigate the nature and pathogenesis of pingueculae using histochemical and immunohistochemical staining.
Methods
Fifty pingueculae in 40 patients were included in this study. AF of the pingueculae was observed and analyzed using a cobalt-blue filter with an additional yellow filter on a slit-lamp. Hematoxylin-eosin and immunohistochemical stainings were performed on surgical specimens of pingueculae that were prepared from each patient. Immunohistochemical staining included Congo red, Oil Red O, periodic acid-Schiff (PAS), Masson's trichrome, transglutaminase-2 (TG-2), mesenchymal stem cell markers CD29 (β-1-integrin), and CD34.
Results
AF images revealed hyper-AF in the pinguecula area. The AF lesions of pingueculae showed superficial punctuate erosions and avascular lesions. Deposition of eosinophilic and amorphous materials in the subepithelial layer of the pinguecula were observed on hematoxylin-eosin staining. Historeactivities to Congo red, PAS, Oil Red O, alcian blue, and Masson's trichrome were not detected, but immunoreactivities to CD29, CD34, and TG-2 were detected in the pingueculae with AF. However, CD29, CD34, and TG-2 were not detected in the pingueculae without AF.
Conclusions
The AF of pingueculae may be related to CD29, CD34, and TG-2. We suggest that pingueculae with AF have a different pathogenesis compared to pingueculae without AF.
A pinguecula is a yellowish to brown protruding lesion in the conjunctiva that is easily seen on the nasal and temporal sides of the cornea. Pingueculal development is affected by a number of intrinsic and extrinsic factors including patient's age and levels of sun, wind, and dust exposure. The full nature and pathogenesis of pinguecula, however, remain unknown [1]. Pingueculae also exhibit features associated with p53 mutation and increased cholesterol metabolism, and comprise a potentially proliferative tissue [2]. Dong et al. [3] reported that pingueculae had abnormal epithelial differentiation of squamous proliferation and metaplasia.
Fluorescence describes the ability of certain molecules to emit light energy of a longer wavelength when stimulated by light of a shorter wavelength. On the other hand, autofluorescence (AF) describes the emission of fluorescence without fluorescent dye in the eye. Recently, fundus AF has been used in a variety of retinal disorders [4-8], with origination from lipofuscin in the retinal pigment epithelium (RPE).
Utine et al. [9] reported that pingueculae display hyper-AF on confocal scanning laser ophthalmoscopy. The actual pinguecula size can be estimated by AF characteristics, and is generally larger than the visible lesion size. However, this report did not include histopathological examinations of autofluorescent pingueculae. Additionally, they did not report the correlation of pinguecula pathogenesis and AF.
In this study, we aimed to analyze the AF properties and investigate the nature and pathogenesis of pingueculae using histochemical and immunohistochemical staining.
Materials and Methods
Patients
Among those who visited our clinic (Chung-Ang University Hospital), patients with pingueculae who displayed AF without any ocular surface disorders were included in this study. Informed consent was obtained from all participants (IRB no. 2008-023-07). We examined AF using a cobalt blue filter with an additional yellow filter on a slit lamp biomicroscope. Forty pingueculae that exhibited AF were excised from 30 patients and 10 pingueculae without AF were excised from 10 patients.
Hematoxylin-eosin staining and immunohistochemistry
In each case, all specimens were formalin-fixed and paraffin-embedded. In order to observe the general composition and topohistological characteristics of the pingueculae, 3-5-micron-thick samples were stained by classic histochemical methods: Hematoxylin-eosin, Alcian blue, periodic acid-Schiff (PAS, for carbohydrates), Oil Red O (for lipids), Masson's trichrome (for connective tissue), and Congo red (for amyloid). Human monoclonal antibodies CD29 and CD34 were used to identify mesenchymal stem cells by immunohistochemical staining. Transglutaminase-2 (TG-2) is known to play a crucial role in wound healing, cell migration, apoptosis, and maintenance of the ocular surface [10]. Therefore, we performed TG-2 immunohistochemical staining for assessing the wound healing process. Based on staining patterns, we graded each slide according to the intensity of staining: negative (-), weak (±), or positive (+).
Results
A total of 50 eyes from 40 patients were included in the study. There were 28 male and 22 female patients. The mean age of the patients was 63.5 ± 7.2 years (range, 45 to 78 years). On slit-lamp examination, pingueculae with AF were observed in two patterns. One pattern was a round and elevated lesion, and the other pattern was flat with bizarre, irregular margins. Round and elevated pingueculae have stronger AF intensity, dilated tortuous vessels, no vessel invasion and focal erosions. Bizarre and flat pingueculae have a scattered AF shape, multiple vessel invasion and diffuse erosions (Fig. 1). Examples of hematoxylin-eosin staining and immunohistochemistry are shown in Fig. 2. In all pinguecula specimens, deposition of eosinophilic and amorphous materials in the subepithelial layer of the conjunctiva and degeneration of the collagen fibers of the conjunctival stroma with thinning of the overlying epithelium and calcification were observed (Fig. 2).
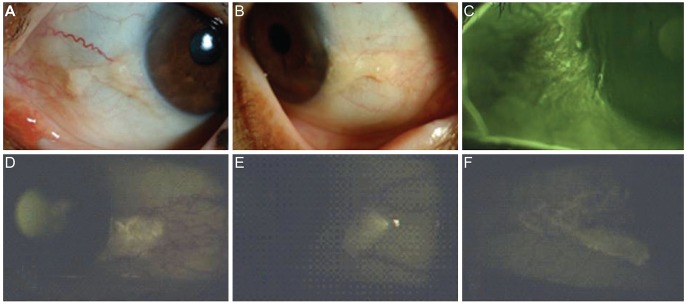
(A,B) Dilated tortous vessel, no vessel invasion and whitish deposits were seen in the pinguecula area. (C) Multiple erosions were observed in the area of pinguecula autofluorescence (AF). (D-F) Various images of pingueculae AF acquired using a cobalt blue filter with an additional yellow filter on a slit lamp biomicroscope.

Immunohistochemical staining of pinguecula autofluorescence (AF). (A) Conjunctiva stroma with thinning of the overlying epithelium and calcification were detected. (B) Eosinophilic-stained amorphous material was detected in the subepithelial layer of the conjunctiva. (C-F) No immunoreactivity was detected. (G) Immunoreactivity to transglutaminase-2 was detected in the normal epithelium, normal vessel walls, and elastotic degeneration area of AF. (H,I) Immunoreactivity was detected. (A,B) Hematoxylin-eosin, (C) periodic acid-Schiff, (D) Masson's trichrome, (E) Congo red, (F) Oil Red O, (G) transglutaminase-2, (H) CD29 (β-1-integrin), and (I) CD 34 (×400).
Positive immunoreactivity to TG-2, integrin (CD29), and CD34 was detected in the subepithelial layer in 17 of 40 pingueculae with AF, but no immunoreactivity was detected in the pingueculae without AF. By contrast, no immunoreactivity to PAS, Masson's trichrome, Congo red, or Oil Red O was detected in any pinguecula (Fig. 2).
Discussion
In this study, we imaged the AF of pingueculae using a cobalt blue filter with an additional yellow filter on a slit lamp biomicroscope. Recently, Utine et al. [9] demonstrated that hyper-AF originates from pingueculae using confocal scanning laser ophthalmoscopy. They compared the anterior images to hyper-fluorescence areas. The authors proposed that either lipofuscin may be associated with the subconjunctival degenerative process of pingueculae, or that the hyper-AF of pingueculae may be related to an undetermined fluorophore.
In fundus AF (FAF) imaging, various tools including color fundus photographs and angiography were used. With the advent of confocal scanning laser ophthalmoscopy, it is possible to visualize FAF and its spatial distribution [11-13]. Using spectrophotometric investigations, Delori et al. [14] showed that lipofuscin granules in the RPE cell monolayer contain the dominant fluorophores responsible for FAF imaging. However, our system is based on slit lamp examination, a new, easy, fast, low-cost method of examining patients with pinguecula on AF imaging.
The main part (mass) of the pterygium is composed of connective tissue that, over the course of pterygium evolution, undergoes pathological changes that are accompanied by an increase of the mass of the pterygium itself. The newly formed structure in the pinguecular part of the pterygium is located subepithelially and progresses towards the cornea [15]. We suggest that the flat, bizarrely shaped pingueculae had characteristics of slow progressive pterygium.
Histological examination has not fully demonstrated the nature of pingueculae. However, all of the analyzed pingueculae on AF images could be defined as elastotic degenerations. Hogan and Alvarado [16] found that elastotic material within the pterygium is formed in four ways: 1) by degenerated collagen, 2) by degeneration of pre-existing elastic fibers, 3) by abnormal fibroblastic activity, and 4) by ground substance disorder. Austin et al. [17] reported elastotic degeneration only under abnormal fibroblastic activity with the production of abnormal maturational forms of elastic fibers. In addition, they found that the pinguecular fibroblasts might have a role in formation of abnormal elastic fibers. Raizada and Bhatnagar [18] suggested that pinguecula was a precursor of pterygium on the evidence of the histopathology of pinguecula. They suggested that histopathological findings of pingueculae resembled in many respects the late fibrotic or early atrophic sclerotic phase of pterygium.
Zhang et al. [10] reported that TG-2 plays a crucial role in wound healing, cell migration, apoptosis, and maintenance of the ocular surface. Our results showed that TG-2 immunoreactivity was positive in pingueculae with AF, but negative in pingueculae without AF. Additionally, the CD29 and CD34 immunoreactivity in pingueculae with AF suggests the possibility that fibroblasts are involved. Therefore, we suggest that pingueculae with AF have a different, more aggressive pathogenesis than those without AF.
The present study has a limitation in that the percentage of positive reactivity to TG-2, CD29, and CD34 was 47.5% in the samples of pingueculae with AF. However, the difference was obvious compared to pingueculae without AF. Additional research is needed to determine the exact pathogenesis in pingueculae with AF.
Notes
No potential conflict of interest relevant to this article was reported.