Choroidal Venous Pulsations at an Arterio-venous Crossing in Polypoidal Choroidal Vasculopathy
Article information
Abstract
It has been reported that pulsations in abnormal vessels are observed on indocyanine green (ICG) angiography in half of patients with polypoidal choroidal vasculopathy (PCV), although the mechanism of the pulsation is unknown. In this study, we report a case of PCV showing venous pulsations at an arterio-venous (A-V) crossing, and discuss a possible mechanism of polypoidal vessel formation and pulsations in PCV. A 66-year-old female presented with a reddish-orange elevated lesion and serous retinal detachment in the macula of her left eye, and was diagnosed as PCV. She was treated with photodynamic therapy (PDT), and followed-up through routine examinations, including ICG angiography. ICG angiography at presentation showed a branching vascular network and choroidal venules with dye leakage (polypoidal vessels) in the left eye. Pulsations, supposedly of venous origin, were observed at an A-V crossing in the abnormal vessels. Within 3 months after PDT, the polypoidal vessel ceased to leak and the pulsations vanished. The reddish-orange lesion gradually decreased in size with complete disappearance of retinal detachment. This study suggests that an unusual compression at an A-V crossing may make a venule polypoidal, and fluctuations of blood flow and pressure in the venule may cause pulsatile movements of the vessel wall.
Polypoidal choroidal vasculopathy (PCV) is characterized by reddish-orange elevations in the peripapillary and/or macular areas showing a polypoidal vessel and a branching vascular network. The mechanism of formation of these structures is controversial. Indocyanine green (ICG) angiography is useful to detect these abnormal vascular elements. In addition, ICG angiography reveals pulsatile movements, which have not been reported in any other chorioretinal diseases, in abnormal vessels in 50% of patients with PCV [1,2]. The frequent occurrence of this pulsation suggests that it is significant, though its presence may go unnoticed or be underestimated. Here, we report a PCV eye demonstrating choroidal venous pulsations at an arterio-venous (A-V) crossing adjacent to the polypoidal lesion, and discuss a possible mechanism of polypoidal vessel formation and pulsation.
Case Report
A 66-year-old Japanese woman was referred to us with a 6-month history of decreased vision and metamorphopsia in the left eye. Visual acuity was 20 / 16 for the right eye and 20 / 32 for the left eye. Fundus examination showed mild epiretinal membrane in the macula of the right eye, and an elevated, oval, 1-disc-diameter, reddish-orange lesion accompanied by serous detachment of the neurosensory retina in the macula of the left eye (Fig. 1A). During the choroidal arteriolar filling phase of ICG angiography (HRA2; Heidelberg Engineering, Heidelberg, Germany), branching vessels (arterioles) appeared in blocked fluorescence, and a few dilated choroidal venules with dye leakage manifested in the early choroidal venular filling phase (Fig. 1B). The dye proceeded extremely slowly in the choroidal venules, leaking to form polypoidal vessels. Notably, the early choroidal venular filling phase revealed pulsatile movements as subtle rhythmic variations in the caliber of the choroidal venule at an A-V crossing (Fig. 1B, yellow arrowhead), where the venule (Fig. 1B, blue arrowheads) crossed over the arteriole (Fig. 1B, red arrowheads). In the later choroidal venular filling phase, the polypoidal lesion filled with dye (Fig. 1C). Her left eye was diagnosed as PCV and treated with photodynamic therapy using verteporfin (Visudyne; Novartis AG, Basel, Switzerland) according to the standard protocol (698-nm laser system, 50 J/mm2, 83-s exposure time, 2,650 µm spot size). Within 3 months after treatment, the polypoidal vessel ceased to leak and the pulsation vanished (Fig. 2B). The reddish-orange lesion gradually decreased in size with complete disappearance of the retinal detachment (Fig. 2A).
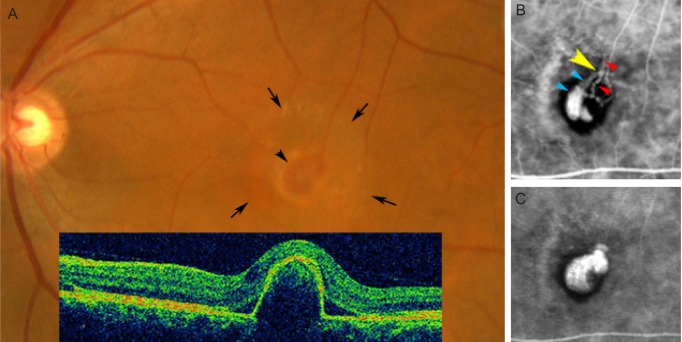
(A) Color fundus and optical coherence tomographic images (inset) of the left eye at the first presentation demonstrating an elevated reddish-orange lesion (arrowhead) associated with serous detachment of the neurosensory retina (arrows). (B,C) Sequential indocyanine green angiograms. Twenty-six seconds (B), and 234 seconds (C) after dye injection. (B) In the early choroidal venular filling phase, a few dilated choroidal venules with dye leakage (blue arrowheads) appear. A pulsatile movement is seen at an arterio-venous crossing (yellow arrowhead) where the venule crossed over the arteriole (red arrowheads). (C) In the later choroidal venular filling phase, the polypoidal lesion is filled with dye corresponding to the reddish-orange lesion.

(A) Color fundus photograph and optical coherence tomographic image (inset) of the left eye 3 months after the photodynamic therapy demonstrating complete disappearance of the retinal detachment. (B) Indocyanine green angiogram revealing disappearance of the polypoidal vessel and pulsation (20 seconds after dye injection).
Discussion
The present findings support the hypothesis that pulsations in PCV might occur at A-V crossings or at A-V shunts, as discussed in our previous study [2]. Importantly, the pulsations disappeared when dye leakage from the polypoidal vessel ceased after treatment, suggesting a relationship between venous pulsations and polypoidal vessel formation.
Regarding venous pulsations, spontaneous venous pulsation (SVP) is well known on the optic disc, where the central retinal vein and artery are fixed to the bundle of the lamina cribrosa by dense collagen and elastic tissues, resulting in the vessel lumen being narrow at this location, and accounting for the greater frequency of occlusion at or near this site. The SVP is observed as a subtle narrowing and expansion of the retinal veins. SVP is generally believed to be caused by the oscillation of intraocular pressure, which is transmitted to the intraocular central retinal vein across the vessel walls, during the cardiac cycle at a significantly higher level than the pressure in the retrolaminar portion of the central retinal vein [3,4]. This theory may also explain the mechanism of pulsation in our case: The venous pulsations may be caused by oscillations of intraocular pressure transmitted to the choroidal venule during the cardiac cycle when there is a significant difference in intravenous pressure between both sides of the A-V crossing. The cause of the difference in intravenous pressure may be that the choroidal venule is made unusually narrow by the compression at the A-V crossing. In the retina, branch retinal vein occlusion occurs at the A-V crossing portion, particularly in arteriolar sclerosis: A vein compressed by an artery is occluded, showing stasis and dilation in the portion proximal to the A-V crossing [5]. In the present case, a similar mechanism might contribute to the venous stasis and the increase in intravenous pressure, resulting in dilation of the portion proximal to the A-V crossing, that is, the polypoidal vessel, although occlusion did not occur. Consequently, fluctuations in blood flow may occur at an A-V crossing, causing variations in the caliber of the venule. Previous clinicopathologic studies revealing a close relationship between pathologic polypoidal choroidal venules and arterioles support this possibility [6,7].
This study suggests that unusual compression at A-V crossings may make the venule polypoidal, and fluctuations of blood flow and pressure in the venule may cause pulsatile movements in the vessel wall. Further studies elucidating the relationships between pulsation, A-V crossings, and polypoidal vessels would be helpful in understanding the pathogenesis of the formation of polypoidal vessels.
Notes
No potential conflict of interest relevant to this article was reported.