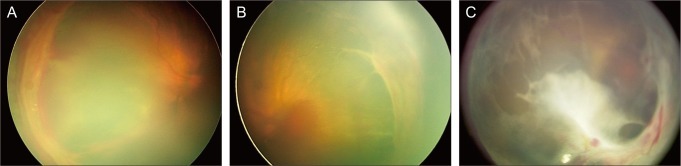
Capone and Trese [
1] first suggested lens-sparing vitrectomy (LSV) to reduce the progression of disease and improve the patient's visual prognosis for macula-sparing (stage 4A) retinopathy of prematurity (ROP)-related retinal detachment. Since then, LSV has been used widely in stage 4 or 5 ROP and has shown favorable anatomic and visual outcomes, especially in stage 4 ROP [
2-
12]. LSV has certain advantages over the other surgical procedures used to treat ROP; it does not cause a myopic shift and does not require additional surgery for buckle removal as compared with scleral buckling (SB), and the post-LSV phakic condition that aids visual rehabilitation is advantageous to the aphakic condition after lensectomy and vitrectomy (LV). Potential disadvantages of LSV compared with LV include less effective relief of anterior traction when the lens is spared. In cases of aggressive posterior ROP (APROP) [
13], LSV is unsuccessful because of insufficient removal of the vitreous gel in the vitreous base [
14]. Therefore, LSV is most suitable for ROP in which tractional retinal detachment (TRD) is located in the posterior retina and when the fibrovascular tissue does not extend to the lens.
In 2006, we reported the anatomic and visual results of LSV for stage 4 and 5 ROP in Korean infants [
5]. In that report, we suggested that LSV produced encouraging surgical outcomes in the correction of ROP-related retinal detachment, especially in stage 4A ROP eyes, after a mean post-operative follow-up period of 2.2 years. Since then, in our consecutive cases with stage 4A ROP showing a limited vascularization in the zone I or zone II posterior retina and rapidly progressive, fovea-threatening TRD with extensive tractional membrane and/or vascular activity, LSV has been unsuccessful. We defined these cases as progressive posterior-type stage 4A ROP, including APROP.
To date, the papers reporting favorable outcomes after LSV for stage 4A ROP [
1-
5,
9-
11] include few cases of this particular-type of ROP. Because the surgical outcomes of progressive posterior-type stage 4A ROP were different from those of previous studies [
1-
5,
9-
11], we investigated the clinical characteristics, long-term results, and associated complications of LSV and possible risk factors for surgical failure in a series of consecutive cases of progressive posterior-type stage 4A ROP.
Materials and Methods
The medical records of all eyes that underwent LSV for stage 4A ROP were reviewed retrospectively at the Department of Ophthalmology, Seoul National University Children's Hospital, Seoul, Korea between January 1999 and December 2007. We included eyes with stage 4A ROP in which retinal vascularization was limited to the zone I or zone II posterior retina. The patients who had been enrolled in the previous study [
5] were included in this study. The follow-up period after LSV exceeded 24 months in all eyes. The stages of ROP were defined in accordance with the international classification of ROP and plus disease (vascular dilation and tortuosity in at least 2 quadrants of the posterior pole) [
13,
15]. Written informed consent was obtained from the parents of all participants before examination under anesthesia and LSV. This study and data collection conformed to all local laws and were performed in adherence to the principles of the Declaration of Helsinki.
The decision to perform surgery and all surgical procedures were made by an experienced pediatric retinal surgeon (YSY). In eyes with stage 4A ROP, LSV was performed only in cases that had a fovea-threatening, posteriorly-located, and progressing TRD and in which fibrovascular tissue did not reach the vitreous base. In eyes with non-progressive TRD, surgery was not performed because it was unnecessary. In an eye with progressive TRD that was located more peripherally, SB was performed instead of LSV. The surgical techniques for LSV were the same as those described by Maguire and Trese [
16] using a 2-port system pars plicata approach. Core vitrectomy was conducted with a vitreous cutter. Organized vitreous stands and tractional membranes were removed by careful delamination and segmentation using a microvitreoretinal blade, spatula, vitreous scissors, and cutter. The surgery was complete when as much of the tractional membrane as possible was removed without injury to the lens and when the entire retina was free from traction. The extent of surgery varied widely depending upon the configuration of the detachment and extent and location of the tractional membrane. However, to prevent iatrogenic tear, membranes that adhered to the retina too tightly to be separated were left in place after relieving traction with segmentation. If there was intraoperative bleeding, endodiathermy was applied until the bleeding was controlled. Intra-vitreal air injection was performed at the end of the surgery in order to maintain eyeball pressure.
All patients were examined in the clinic after the operation, and visual rehabilitation was performed according to individual needs. Follow-up examinations were performed periodically with special emphasis on monitoring the retinal status and vision, as well as identifying complications such as intraocular hemorrhage, cataract, corneal opacity, secondary pupillary-block glaucoma, microphthalmia, and strabismus. All procedures and follow-up examinations were performed by the operating surgeon.
Data collected from each case record included gender, gestational age at birth, birth weight, treatment of ROP before LSV, and gestational age at LSV. ROP findings included the preoperative ROP stage, zone, severity of plus disease at LSV, and extent of fibrovascular proliferation and traction. ROP findings were recorded by detailed retinal drawings and/or fundus photographs. The severity of plus disease was defined as absent (there was no vascular dilation or tortuosity at the time of surgery), mild to moderate (there was obvious vascular dilation and tortuosity of the posterior pole [
13]), or severe (there was more severe vascular dilation and tortuosity than the standard photograph [
13]). Further, we collected treatment histories for retinal detachment, post-LSV results including anatomic results, visual acuity, prevalence of any complications, and follow-up period after LSV.
Anatomical success was defined as total reattachment of the retina or at least posterior polar reattachment within the arcades and included those eyes with persistent peripheral retinal fold as long as the posterior pole showed attachment [
5,
8]. Final visual acuity was classified into the following categories: favorable vision, which included ambulatory vision (visual acuity better than 20 / 1900) [
7] and form vision (patients can fix their eyes on and follow a small moving toy); and unfavorable vision, such as light follow (patients can fix their eyes on and follow a moving penlight), light perception (patients can differentiate between the light being on and off), and no light perception [
5].
Results
A total of 11 eyes of 9 patients (5 girls and 4 boys) underwent LSV for progressive posterior-type stage 4 ROP. The mean gestational age at birth of the patients was 27 weeks 6 days (range, 25 weeks 4 days to 31 weeks 6 days), and the mean birth weight of the patients was 1,113 g (range, 780 to 1,400 g).
Table 1 lists the demographics and preoperative retinal status of the cases included in this study. Cases 1 to 4 had been included in the previous study by Yu et al. [
5]. Ten eyes were treated at least once with peripheral ablation using diode laser photocoagulation to the avascular retina before LSV. One patient (case number 1) underwent cryotherapy before diode laser photocoagulation because of poor pupillary dilation and vitreous haziness. After cryotherapy in that patient, pupil dilation and vitreous haziness improved, and diode laser photocoagulation was performed in that eye. All treated eyes progressed to TRD and underwent LSV. One patient (patient number 5) presented with severe extraretinal fibrovascular proliferation and retinal traction threatening macula at the first examination and was initially treated with LSV.
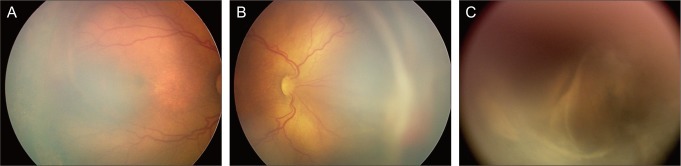
The mean gestational age at LSV was 39 weeks 6 days (range, 35 weeks 6 days to 44 weeks 5 days). At the time of LSV, 6 eyes had retinal vascularization to zone II posterior area and the rest to zone I, 4 of which presented as APROP. Although plus disease had regressed after previous peripheral laser ablation in 3 eyes, it was still moderately active in 5 eyes and very active in the other 3 eyes at the time of surgery. In all eyes with APROP, plus disease did not regress even after repeated peripheral ablation treatments. Active fibrovascular proliferation was present in all eyes, and extensive tractions in more than 6 clock-hours were observed in 7 eyes, including 3 eyes with almost 360 degrees of active circumferential tractional membranes.
Although the traction membranes were strongly attached to the retina in all eyes, iatrogenic retinal tears did not occur in all eyes because of careful delamination and segmentation of tractional membrane, which is described in the methods section. Intraoperative bleeding was controlled with endodiathermy during surgery, and there was no grossly observed intraocular bleeding at the end of the surgery.
The surgical outcomes of LSV are shown in
Table 2. After LSV, no retinal tears, lens injuries, or cataracts were found during the immediate postoperative period in any eyes. However, mild preretinal hemorrhage (PRH) developed in 3 eyes, and severe PRH developed in 2 eyes. Mild vitreous hemorrhage (VH) was detected in 4 eyes, while severe VH was detected in only 1 eye. Three eyes did not present any immediate postoperative intraocular hemorrhage. In 8 eyes that presented with preoperative plus disease, 6 (75%) demonstrated postoperative intraocular hemorrhage such as PRH and VH. On the other hand, in 3 eyes with regressed plus disease, 2 (66%) showed intraocular hemorrhage after surgery. Statistical analysis between the groups with and without plus disease at the time of LSV was impossible due to the small number of cases. However, all eyes with APROP with plus disease developed severe PRH or VH after LSV.
Anatomic reattachments of the retina were attained in 6 eyes (54.5%) after single LSV. Four eyes (cases 3, 5, 8 and 9) had total reattachment of the retina, and 2 eyes (cases 4 and 10) had posterior polar retinal reattachment within the arcades, although peripheral detachment was present. In eyes with total reattachment, total TRD recurred in 2 (cases 5 and 9) of 4 eyes, at postoperative 2 and 10 months, respectively. One eye (case 5) showed retrolental fibroplasia. In 2 eyes with posterior polar reattachment, macular detachment occurred at postoperative 7 and 40 months, respectively.
LV was performed in 4 eyes, including 2 (cases 2 and 6) that showed no reattachment after LSV and 2 eyes (cases 4 and 10) that showed initial reattachment but had recurrence of TRD during the follow-up exam. In 1 eye (case 9), LV was attempted at the time of the second surgery but was impossible due to corneal opacity. In the rest of the unsuccessful cases, additional surgery such as LV was not performed because corneal opacity obstructed the vitreous surgery or the retina was too stiff to expect success after LV. We also considered the retinal status of the opposite eye, and additional vitrectomy was chosen when the benefits of surgery outweighed the costs. After LV, retinal reattachment was obtained in 2 eyes (cases 2 and 4), of which TRD recurred again in 1 eye (case 2) at 36 months after LV. Therefore, there were 3 eyes (cases 3, 4 and 8; 27.3%) that had retinal reattachment after multiple surgeries, consisting of 2 eyes (66%) without plus disease and 1 eye (13%) with plus disease. Although statistical analysis between the groups was impossible, the eyes without plus disease at the time of surgery might have had more opportunities for retinal reattachment.
During the follow-up period, recurrent TRD developed in 5 of 7 eyes that had retinal attachment with LSV or LV. When surgery for ROP was unsuccessful, development of cataract, corneal opacity, or glaucoma was common. However, when surgery for ROP was successful, these complications were not observed. One eye with an attached retina showed retinal atrophy (
Table 2), and lensectomies were performed in 3 eyes due to secondary pupillary-block glaucoma or cataract. Strabismus developed in 2 patients, one showed normal posterior polar fundus in the opposite eye, and one had mild retinal dragging in the opposite eye.
The mean postoperative follow-up period of the patients was 4.6 years (range, 2.8 to 7.8 years), and the mean age of the patients at the final follow-up was 5.0 years (range, 3.0 to 8.0 years). At the final examination, 2 of the eyes with reattached retinas had favorable visual outcomes, and 1 eye had unfavorable vision of light follow (the ability to fix on and follow a moving penlight). On the other hand, in eyes with detached retinas, all eyes had unfavorable visual outcomes including 5 eyes with no light perception, 2 with light perception, and 1 with light follow.
Discussion
In the present study, progressive posterior-type stage 4A ROP showed a poor anatomical success rate after LSV, which was different from the results of previous reports [
1-
12]. In a previous study [
5], the success rate of stage 4A ROP eyes was 75% after a mean post-operative follow-up period of 2.2 years; however, in the present study, the overall success rate of LSV for the same stage ROP was 27% after a mean follow-up period of 4.6 years.
One of the reasons for this poor result is that the operation was performed only in eyes with stage 4A ROP that showed retinal vascularization to the zone I or zone II posterior retina and progressing TRD with extensive fibrovascular proliferation. The eyes with stage 4A ROP with progressive TRD located on the more peripheral retina usually underwent SB or additional laser photocoagulation around the traction. Moreover, eyes with stage 4A ROP with TRD that did not progress and that did not have vascular activity were observed regularly with no need for further treatment. In other studies that demonstrated favorable anatomic success rates for stage 4A ROP [
1-
12], LSV was usually performed in the eyes with peripheral traction anterior to the equator after the vascular activity had regressed. According to our experience, posterior-type stage 4A ROP with vascular activity tended to progress rapidly to stage 4B or 5, resulting in surgical failure and blindness. We performed LSV in these eyes before the macula became detached. Although this study demonstrated that the anatomic results of LSV in progressive posterior-type stage 4A ROP are poor, some of the eyes were saved by this procedure.
It is important to select appropriate cases in order to ensure the surgical success of LSV. Hartnett [
17] reported that the features associated with poor surgical outcome were vitreous haziness, neovascularization, and plus disease. It is possible that plus disease had an adverse effect on the surgical outcomes in our study. The success rate in eyes without plus disease at the time of LSV was 66%, whereas the rate in eyes with plus disease was 13%. In all cases of APROP with pre-operative plus disease, LSV failed to reattach the retinas. Although statistical analysis was impossible in our study due to the small number of cases, the presence of plus disease seemed to negatively affect the surgical results. Therefore, LSV should be performed carefully in eyes with vascular activity.
In addition to plus disease, a limited area of vascularized retina may also be a main cause of surgical failure in progressive posterior-type stage 4A ROP. Fibrovascular tissue grows close to the macula and rapidly progresses toward the posterior retina, leading to macular detachment. In cases of APROP, early surgical intervention was recommended before traction was initiated [
14]. According to our experience, posterior-type ROP with zone I or II posterior vascularization also showed rapid progression to stage 4B or 5, similar to APROP, once fibrovascular traction was initiated. Moreover, extensive fibrovascular proliferation accelerated the progression of TRD, resulting in poor surgical outcome. Therefore, earlier surgical intervention might have resulted in better anatomic outcomes in our cases.
LV rather than LSV was recommended in cases of APROP because LSV did not halt the progression of retinal detachment due to insufficient removal of the vitreous gel in the vitreous base [
14]. Because some of the recurred cases in our study showed retrolental fibroplasias, initial lens removal and sufficient removal of the vitreous in the vitreous base might be helpful to prevent recurrent TRD and secondary pupillary-block glaucoma in these cases. Nevertheless, we think initial lens removal is not necessary in most cases in which traction membranes are posterior to the equator and do not extend to the lens. However, the effect of initial lens removal in posterior-type stage 4A ROP needs further investigation.
Immediate postoperative VH or PRH may also be considered as a cause of surgical failure because retinal reattachment was not attained in all eyes with severe VH and/or PRH in this study. There are many growth factors in the blood, and the release of growth factors from intraocular bleeding might promote postoperative fibrovascular proliferation, although this requires further investigation. Therefore, delamination of the fibrovascular membrane should be performed carefully, and intraoperative bleeding control and irrigation of intraocular blood should be fully achieved.
A longer follow-up period also contributed to the low anatomic success rate. During the follow-up period, TRD recurred in 5 eyes at variable time intervals from the LSV or LV, ranging from 2 months to 40 months. In addition, Kondo et al. [
18] previously reported that retinal detachment could recur after successful retinal reattachment with LV at variable time intervals. As suggested by the authors [
18], persistent traction is the major cause of the late recurrence of TRD. Therefore, removal of as much of the tractional membrane as possible during the surgical procedure is very important for long-term success. In this study, the traction membranes were strongly attached to the retina in all eyes. We tried to remove as much of the tractional membrane without injury to the lens. In cases where the membranes adhered to the retina too tightly to be separated, tractions were relieved completely with the segmentation technique, though the membranes were left intact. Nevertheless, TRD recurred at variable time intervals due to both reproliferation of fibrous tissue and persistent membrane. Although early recurrence might be caused by the reproliferation of fibrous tissue and late recurrence by persistent traction, the pathology of recurrence was not fully investigated in this study because the interval of recurrence from surgery was variable among the cases, and close follow-ups were not performed after surgery except during the first few post-operative months.
The visual outcomes of LSV in previous studies have been variable [
1-
11], with quite favorable visual outcomes in the stage 4A ROP group [
9-
11]. Lakhanpal et al. [
11] reported that the mean visual acuity was 20 / 62 for stage 4A ROP after LSV. In our cases, visual acuity better than 20 / 1,900 was achieved in only 1 eye. The lower anatomic success rate, as well as the higher prevalence of complications that interrupted visual development, such as corneal opacity, cataract, and glaucoma, are possibly the main causes of these poorer visual outcomes. Although Moshfeghi et al. [
2] reported a low prevalence of complications from LSV, the prevalence of complications was much higher in our cases, especially in eyes with detached retinas. This report demonstrated that retinal reattachment was very important for gaining favorable vision, as well as to prevent long-term complications.
Because the presence of plus disease at the time of surgery is the main risk factor of surgical failure [
17], reduced plus disease activity may be helpful for surgical outcome. The severity of plus disease was related to the area of the vascularized retina. The wider was the avascular retina, the more angiogenic factor was released, which leads to increased vascular activity. In most of our cases, plus disease persisted after repeat diode laser photocoagulation of the nonvascularized retina. In a previous report, a wide-field area of hypoperfusion was detected in a nonvascularized retina and in a vascularized retina in APROP [
19]. Moreover, application of photocoagulation to vascularized and nonvascularized retinas was suggested to reduce the recurrence of fibrovascular tissue in APROP [
20]. In cases of posterior-type ROP, retinal hypoperfusion of the vascularized retina might be present. Therefore, photocoagulation of the vascularized retina might help regress plus disease in these cases. Recently, intravitreal injection of anti-vascular endothelial growth factor (VEGF) antibody was highlighted as a new therapeutic modality in the treatment of ROP and has been used as monotherapy or as combination therapy with laser or vitrectomy. Kusaka et al. [
21] and Law et al. [
22] suggested that intravitreal bevacizumab, when it was injected before or after definite laser or surgical treatment, may decrease vascular activity and facilitate disease regression in eyes with ROP at high risk for progression. In our cases, early surgery might have been beneficial before the traction was initiated, but vascular activity at the time of surgery may have been a risk factor of surgical failure. From this point of view, intravitreal anti-VEGF antibody treatment can be considered as an adjunctive treatment before LSV for earlier surgery in eyes with rapidly progressing stage 4A ROP with plus disease. However, further investigations are necessary to study the benefits and side effects of anti-VEGF antibody in eyes with ROP.
As we pointed out, the results of this study were contradictory to those of our previous paper [
5]. Cases 1 to 4 were included in the previous study. The retina in case 2 had been reattached at the time of the previous study, after the second operation, but it subsequently redetached. Therefore, the success rate of 75% in 2006 decreased to 50% in the current study. Although this was a retrospective study, most cases of stage 4a ROP underwent SB or laser only, rather than vitrectomy, and only cases with progressive posterior-type stage 4a ROP underwent LSV in our clinic. Therefore, there was a difference between the case selection in this study and that in the previous study. The discrepancy in results between the two studies might be caused by the follow-up period (2.2 vs. 4.6 years) and the small number of cases.
This study had several limitations. As it was a retrospective and noncomparative consecutive case series, there were only a few cases with severe retinal tractions among the eyes with stage 4A ROP. Therefore, the surgical results of LSV were biased to be insufficient for stage 4A ROP in this study and the results of this study cannot be compared to those of other studies. Moreover, the follow-up visual acuity measurements were not obtained in a systematic fashion. In addition, the follow-up period for the patients varied. However, the strengths of the study are that it is a single-surgeon, consecutive case series conducted over a 10-year period in which all patients were observed for at least 24 months postoperatively.
In this study, we intended to report our experiences with progressive posterior-type ROP that was refractory to laser treatment and rapidly progressed to TRD with extensive fibrovascular proliferation but could not be treated with SB. In conclusion, the anatomic success rate of LSV for progressive posterior-type stage 4A ROP was very low, especially in the presence of plus disease at the time of LSV, and the visual outcomes were not favorable after long-term follow-up. Complications developed frequently during the longer follow-up period, especially in eyes with persistent TRD. Anatomical reattachment is very important for preventing complications and gaining better visual outcomes. Intensive laser treatment on the nonvascularized as well as vascularized retina should be performed in eyes with progressing posterior-type ROP with active plus disease. If the condition continues to progress, adjunctive intravitreal injection of anti-VEGF antibody and early vitreous surgery can be considered to hinder TRD.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Capone A Jr, Trese MT. Lens-sparing vitreous surgery for tractional stage 4A retinopathy of prematurity retinal detachments.
Ophthalmology 2001;108:2068-2070.


2. Moshfeghi AA, Banach MJ, Salam GA, Ferrone PJ. Lens-sparing vitrectomy for progressive tractional retinal detachments associated with stage 4A retinopathy of prematurity.
Arch Ophthalmol 2004;122:1816-1818.


3. Hubbard GB 3rd, Cherwick DH, Burian G. Lens-sparing vitrectomy for stage 4 retinopathy of prematurity.
Ophthalmology 2004;111:2274-2277.


4. Lakhanpal RR, Sun RL, Albini TA, Holz ER. Anatomic success rate after 3-port lens-sparing vitrectomy in stage 4A or 4B retinopathy of prematurity.
Ophthalmology 2005;112:1569-1573.


5. Yu YS, Kim SJ, Kim SY, et al. Lens-sparing vitrectomy for stage 4 and stage 5 retinopathy of prematurity.
Korean J Ophthalmol 2006;20:113-117.



6. Micelli Ferrari T, Furino C, Lorusso VV, et al. Three-port lens-sparing vitrectomy for aggressive posterior retinopathy of prematurity: early surgery before tractional retinal detachment appearance.
Eur J Ophthalmol 2007;17:785-789.


7. El Rayes EN, Vinekar A, Capone A Jr. Three-year anatomic and visual outcomes after vitrectomy for stage 4B retinopathy of prematurity.
Retina 2008;28:568-572.


8. Bhende P, Gopal L, Sharma T, et al. Functional and anatomical outcomes after primary lens-sparing pars plana vitrectomy for Stage 4 retinopathy of prematurity.
Indian J Ophthalmol 2009;57:267-271.



9. Moshfeghi AA, Awner S, Salam GA, Ferrone PJ. Excellent visual outcome and reversal of dragging after lens sparing vitrectomy for progressive tractional stage 4a retinopathy of prematurity retinal detachment.
Retina 2004;24:615-616.


10. Prenner JL, Capone A Jr, Trese MT. Visual outcomes after lens-sparing vitrectomy for stage 4A retinopathy of prematurity.
Ophthalmology 2004;111:2271-2273.


11. Lakhanpal RR, Sun RL, Albini TA, et al. Visual outcomes after 3-port lens-sparing vitrectomy in stage 4 retinopathy of prematurity.
Arch Ophthalmol 2006;124:675-679.


12. Lakhanpal RR, Davis GH, Sun RL, et al. Lens clarity after 3-port lens-sparing vitrectomy in stage 4A and 4B retinal detachments secondary to retinopathy of prematurity.
Arch Ophthalmol 2006;124:20-23.


13. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited.
Arch Ophthalmol 2005;123:991-999.


14. Azuma N, Ishikawa K, Hama Y, et al. Early vitreous surgery for aggressive posterior retinopathy of prematurity.
Am J Ophthalmol 2006;142:636-643.


15. The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. II. The classification of retinal detachment. The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity.
Arch Ophthalmol 1987;105:906-912.


16. Maguire AM, Trese MT. Lens-sparing vitreoretinal surgery in infants.
Arch Ophthalmol 1992;110:284-286.


17. Hartnett ME. Features associated with surgical outcome in patients with stages 4 and 5 retinopathy of prematurity.
Retina 2003;23:322-329.


18. Kondo H, Arita N, Osato M, et al. Late recurrence of retinal detachment following successful vitreous surgery for stages 4B and 5 retinopathy of prematurity.
Am J Ophthalmol 2009;147:661-666.e1.


19. Yokoi T, Hiraoka M, Miyamoto M, et al. Vascular abnormalities in aggressive posterior retinopathy of prematurity detected by fluorescein angiography.
Ophthalmology 2009;116:1377-1382.


20. Yokoi T, Yokoi T, Kobayashi Y, et al. Risk factors for recurrent fibrovascular proliferation in aggressive posterior retinopathy of prematurity after early vitreous surgery.
Am J Ophthalmol 2010;150:10-15.e1.


21. Kusaka S, Shima C, Wada K, et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study.
Br J Ophthalmol 2008;92:1450-1455.


22. Law JC, Recchia FM, Morrison DG, et al. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity.
J AAPOS 2010;14:6-10.









 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print