 |
 |
| Korean J Ophthalmol > Volume 25(6); 2011 > Article |
Abstract
Purpose
To evaluate the clinical results of proton beam radiation therapy (PBRT) for treatment of retinoblastoma.
Methods
Children with retinoblastoma who were treated with chemotherapy and focal treatment such as brachytherapy and thermotherapy but showed no response or developed recurrences later received PBRT. The PBRT strategy was designed to concentrate the radiation energy to the retinoblastoma and spare the surrounding healthy tissue or organs.
Results
There were three patients who received PBRT. The first patient received PBRT because of an initial lack of tumor regression with chemotherapy and brachytherapy. This patient showed regression after PBRT. The second patient who developed recurrence of retinoblastoma as diffuse infiltrating subretinal seeding was taken PBRT. After complete regression, there was recurrence of tumor and the eye was enucleated. The third patient had unilateral extensively advanced retinoblastoma. Initial chemotherapy failed and tumor recurred. The tumor responded to PBRT and regressed significantly. However, the eye developed sudden multiple recurrences, so we had to perform enucleation.
Retinoblastoma is the most common primary intraocular malignancy in childhood. The incidence is estimated at about 1 in 15,000 to 20,000 live births [1,2]. Since the first retinoblastoma patient was successfully treated with radiation therapy [3], due to the radiosensitive nature of retinoblastoma [4], external beam radiation therapy (EBRT) has been thought to be the first line and major treatment method for retinoblastoma. However, there is a very high incidence of secondary malignant neoplasm among the survivors of heritable retinoblastoma [5-7], as well as cosmetic problems of orbital bone growth retardation in those who received EBRT, particularly in younger children [8]. Therefore, treatment modalities were shifted toward primary systemic chemotherapy for reducing tumor volume initially (chemoreduction) and additional focal treatment such as cryotherapy, thermotherapy, or brachytherapy [9]. These latter methods increased the salvage rate of globes and reduced complications of EBRT [10]. However, those methods cannot replace all the area of EBRT for retinoblastoma.
There has been substantial advancement in radiation therapy techniques, including recently developed intensity-modulated radiotherapy, stereotactic conformal radiotherapy and proton beam radiation therapy (PBRT). Among these, PBRT is the most recently developed and has proved to be the superior radiation therapy technique, providing a greater radiation dose to the target tumor with increased sparing of normal adjacent structures. Lee et al. [11] compared these three radiation techniques for the treatment of retinoblastoma, medulloblastoma, and pelvic sarcoma. Including this report, there are a few papers that analyzed the advantages of PBRT for retinoblastoma with regard to radiation distribution, but these lack clinical results after treatment [11-13].
Here, we present clinical courses of three challenging cases of retinoblastoma which were managed with PBRT.
Three retinoblastoma patients who were diagnosed and treated with chemotherapy and focal treatment in Seoul National University Children's Hospital and received PBRT at the Proton Therapy Center, National Cancer Center (NCC) in Korea between June 2007 and August 2009 were selected for this retrospective study.
The eligibility criteria for inclusion were children with newly diagnosed intraocular retinoblastoma who were initially treated with chemotherapy and focal treatment but showed no response to these strategies from the beginning or who showed favorable response at first but developed recurrence of the tumor during or after the treatment. Treatment was therefore followed by PBRT.
Exclusion criteria included eyes considered to be dangerous without immediate enucleation and eyes that exhibited severe vitreal or subretinal seeding or displayed evidence of tumor invasion into the anterior segment, choroid, optic nerve or extraocular tissue following clinical examination, ultrasonography and neuroimaging methods.
We performed two combination regimens of chemotherapy. One consisted of cisplatin, etoposide, doxorubicin, cyclophosphamide and vincristine and the other was a combination of carboplatin, etoposide and vincristine. Every cycle was repeated each month for one week for a total of 6 to 13 cycles, according to patient condition and tumor status. Examination under anesthesia was done before every cycle of chemotherapy and fundus photos were taken using a RetCam II (Clarity Medical System, Pleasanton, CA, USA). Thermotherapy was performed if possible when tumor volume was considerably reduced to allow focal treatment with the Oculight SLX diode laser (Iridex Corp., Mountain View, CA, USA). If there was no response to chemotherapy initially or if recurrence developed after chemotherapy and focal treatment, then we referred the patient to NCC for PBRT.
A plane computed tomography (CT) scan was taken with a 2-mm slice along the orbital space with or without an eye rotation using a suction cup, depending on the tumor locations. Gross tumor volumes (GTVs), clinical target volumes (CTVs), and planning target volumes (PTVs) were delineated. PTV was CTV + 2 mm, and CTV was designed to cover any retina posterior to the equator with the intent to prevent the development of new foci. Critical structures, such as the ipsilateral lens, orbital bone and soft tissues, optic nerve, lacrimal gland, contralateral eye, temporal and frontal lobes, and pituitary gland, were delineated as organs at risk (OARs). Proton beam planning was performed using a three-dimensional treatment-planning system (Eclipse proton planning system ver. 8.1.2; Varian Medical System, Palo Alto, CA, USA) and dose distributions were determined for each target volume and OARs. Three-dimensional proton plans were performed to give PTV 95% of the prescribed dose. The prescribed doses were 46 cobalt grey equivalents (CGE; CGE = proton Gy x relative biological effectiveness 1.1) to PTV for two patients and 50 CGE to PTV for the one patient who had a large tumor with subretinal tumor seeding. For proton beam planning, the proximal and distal margins for targets were 3 mm, respectively, and the border smoothing and smearing margins were set to 0.5 and 1.2 mm, respectively. A smearing margin of 1.2 mm was given to allow the margin for setup uncertainty. The block margins were set to 5 mm. For all patients, a single beam was used in the gantry room to treat the macroscopic GTV and the microscopic CTV. To achieve maximum dose conformity, all beams were customized to the target, with brass apertures and Lucite compensators fabricated by a computer-driven milling machine, to specifications from the planning program.
During the principal treatment setup, the patient was in the supine position under propofol-based total intravenous anesthesia. For each patient, a thermoplastic mask was fabricated. Daily eye setup and treatment field positioning were checked using the laser beams in relation to the suction cup centered on the cornea. The radiopaque wires built into the silicon suction cup served as an auxiliary marker to the patient's bony anatomy. For every patient, anatomy-based clinical setup was performed and then double-checked using a comparison between the digitally reconstructed radiograph obtained from the plane CT scan and X-rays (digital image positioning system) taken in the treatment room.
A 4-year-old boy was referred with sporadic bilateral ocular retinoblastoma. In the right eye, we observed International Classification of Retinoblastoma (ICRB) group E, Reese-Ellsworth Classification (R-E) group V retinoblastoma, occupying the entire globe. In the left eye, ICRB group B, R-E group IV, a retinoblastoma larger than 8 DD size mass was situated anterior to the equator without subretinal or vitreous seeding. After the immediate primary enucleation of the right eye, chemotherapy with cisplatin, etoposide, doxorubicin, cyclophosphamide and vincristine was initiated and the biopsy results showed choroid and optic nerve invasion over the lamina cribrosa but resection margin was clear. Before the third cycle of chemotherapy, we observed an increase in size of the retinoblastoma in the left eye and topotecan was added to the original chemotherapy regimen. After one cycle of the new regimen, the tumor continued to grow. Brachytherapy with I-125 was performed but there was no change in tumor size for six months then we decided to perform PBRT (Fig. 1A).
PBRT with total dose of 46 Gy delivered in 23 fractions was performed (Fig. 1B). One month after the end of PBRT, the tumor started to shrink, and two months later had decreased further, allowing us to perform thermotherapy with diode laser. After additional thermotherapy, six months after the end of PBRT, the tumor had regressed completely (Fig. 1C).
A 1-year-old girl without any family history of retinoblastoma visited for evaluation of left leukocoria. There were multiple tumors around the optic disc and macula with subretinal seeding classified as ICRB group D, R-E group II in right eye and large peripapillary endophytic mass with total retinal detachment classified as ICRB group B, R-E group V in left eye. Chemotherapy with cisplatin, etoposide, doxorubicin, cyclophosphamide and vincristine was initiated, which led to the regression of multiple tumors, the reattachment of the retina and complete regression finally. Despite the application of thermotherapy to the subretinal seeding area, the lesion grew and new lesions of subretinal seeding inferior to the macula developed. We had to consider EBRT in that case and decided to perform PBRT, considering her age.
PBRT, with total dose of 46 Gy delivered in 23 fractions, was performed (Fig. 2C). One month after completion of PBRT, the subretinal seeding had regressed completely (Fig. 2D and 2E). The patient was tumor-free for seven months. However, vitreous hemorrhage was observed at routine examination under anesthesia after eight months of PBRT. We closely observed the patient and found newly developed small mass developed at the area of previously regressed subretinal seeding. The mass have grown rapidly to near the optic nerve during three months, so we carefully decided to enucleate the eye. Pathology result of the enucleated eye showed dysplastic retina with dystrophic calcification, consistent with near complete necrosis of tumor without invasion of choroid, anterior chamber, sclera and lamina cribrosa.
A 5-year-old boy was presented with left leukocoria. There was sporadic unilateral ICRB group E, R-E group V, massive retinoblastoma with diffuse subretinal seeding and total retinal detachment around huge mass in the left eye (Fig. 3A). Chemotherapy with carboplatin, etoposide and vincristine was started and one cycle of chemotherapy led to reattachment of retina and significant shrinkage of tumor volume with reduced subretinal seeding. But before starting third cycle of chemotherapy there was recurrence of diffuse subretinal seeding especially around optic disc (Fig. 3B). Instead of enucleation, which was refused by the patient's parents, we performed PBRT.
PBRT, with total dose of 50.4 Gy delivered in 28 fractions, was performed (Fig. 3C). At the end of PBRT, all subretinal seeding had disappeared around the optic disc and peripheral retina with further reduced volume of the original tumor (Fig. 3D).
However, three weeks later, during the examination before the planned thermotherapy to treat the residual tumor, an increased volume of the retinoblastoma and multifocal recurrence at the area around the tumor and around almost the entire ora serrata were discovered (Fig. 3E). We decided to perform enucleation carefully after consultation with radiation oncologist and pediatric oncologist. Pathology report described there were tumor involvement of about 40% of retina containing 5% necrosis without invasion of choroid, sclera and lamina cribrosa.
The proton is one of the components of atomic nuclei, as discovered by Ernest Rutherford in 1919. Later in 1946, Robert Wilson was the first one who suggested its application for medical treatment [14]. Since then, several efforts have been made to treat tumors using PBRT [16-18] and in 1992, Croughs et al. [18] reported the first treatment results for PBRT in three retinoblastoma patients.
The major benefits of the PBRT are 1) coverage of the target zone with a uniform dose, 2) a zero dose deep to that target for each beam path, 3) a lower dose proximal to the target [19]. Protons can penetrate tissue toward the target tumor with less energy loss, give uniform radiation to targeted tumor and finally distribute no energy beyond the target. These unique properties of protons can reduce the incidence of several side effects of radiation therapy in retinoblastoma including late occurrence of secondary malignant neoplasm, retardation of tissue growth and atrophic or degenerative changes to the tissue.
The most important goal of treatment for pediatric retinoblastoma is to save the life of the child. The next priority is to save the eyeball and vision. The introduction of chemotherapy could have satisfied both purposes. Since the time when Kingston et al. [9] established the current regimen of chemotherapy, it has been used to reduce tumor volume and make additional focal therapy possible. Using these treatment modalities have not only much improved the ocular salvage rates [10,20] but also have reduced the occurrence of secondary malignant tumors and intracranial neuroblastic tumors, such as pinealoblastoma [21].
Despite the effectiveness of chemotherapy, even in cases of initial total tumor regression with chemoreduction followed by focal treatment, subretinal and vitreous seeds recur [22,23]. In one series, the incidence of recurrent vitreous seeds among those who initially presented was about 50% at five years; the incidence of recurrent subretinal seeds at five years was about 62% [23]. These conditions should be managed promptly and the treatment plans could be various focal treatments if seeding were localized. If the seeding is extensive, it should be managed with EBRT or enucleation. In fact, EBRT should be the only way to save the eye in that case.
We presented undesirable circumstances that any ophthalmic oncologist may meet during the management of retinoblastoma. In case 1, the patient presented with sporadic bilateral retinoblastoma and required enucleation of one eye due to the extensive size of the tumor. Unfortunately, the tumor resisted not only various chemotherapy regimens but also brachytherapy using I-125. EBRT had to be considered. Instead of EBRT, we consider PBRT and it had successively controlled the tumor so that we could save the eye while trying to minimize patient suffering due to the occurrence of secondary malignant neoplasm or facial deformity, which may be induced by EBRT. In case 2, also with sporadic bilateral retinoblastoma, the originally discovered multiple tumors responded well with chemotherapy and additional thermotherapy. Even though multifocal recurrence of the tumor developed during chemotherapy, those were treated well with thermotherapy. However, after completing the thirteen cycles of chemotherapy, a large area of subretinal seeding developed. We tried focal consolidation with diode laser but subretinal seeding was advanced. PBRT made it disappear completely. In case 3, the large sporadic unilateral retinoblastoma, which was presented later than in the other two children, was accompanied by peripheral extensive subretinal seeding. Furthermore, total retinal detachment made us consider primary enucleation. However, the parents stoutly refused this option, so we started chemotherapy as a second option. The tumor seemed to respond well to chemotherapy at first but during the early period of chemotherapy, diffuse subretinal seeding surrounding the optic disc developed. We were afraid of local invasion through the optic disc. Therefore, instead of enucleation, we proceeded with PBRT and the result was favorable; complete resolution of subretinal seeding with quite reduced tumor volume was seen, allowing additional thermotherapy. But three weeks after the completion of PBRT, we obtained unexpected results. Not only did the main tumor grow significantly, but multifocal recurrences also developed around the tumor and at the ora serrata. We had to perform enucleation in the end.
Considering the differences between the two cases of favorable results and the last devastating case, the latter involved disseminated subretinal seeding at presentation, anterior to the equator, with massive tumor. Even if seeding appeared to have disappeared after two cycles of chemotherapy, as Wilson et al. [22] suggested, tumor cells could exist in vitreous or avascular subretinal spaces that the chemotherapy could not reach. EBRT is the treatment of choice for diffuse subretinal and vitreous seeding when systemic chemotherapy has failed. Although the ocular survival rate in advanced retinoblastoma (R-E group Vb) is about 50% with EBRT, the results are not promising [24]. Similar to the case presented here, the original tumor mass grows and multiple tumor recurrences develop around the main mass despite PBRT. That means the tumor had characteristics of resistance not only to chemotherapy agents but also to radiation. Another consideration in this case was the fact that multiple recurrent tumor foci located near the ora serrata might be related to the method of radiation distribution for sparing lenses. The more anterior part, from equator to ora serrata, was involved in the radiation field where higher cataracts developed [25]. To avoid the development of cataracts, the posterior part of the ora serrata was excluded from the radiation field so there were higher recurrence rates at the anterior retina, especially the ora serrata, as observed in this case.
In conclusion, PBRT was a effective initially in cases of late recurrence, such as diffuse subretinal seeding after completion of chemotherapy or no response to chemoreduction with additional focal treatment including brachytherapy. However, as the case 2 and 3 demonstrate, even when retinoblastoma seemed to respond to PBRT, it could recur after a few months. Therefore, we need to observe patients who received PBRT and demonstrated favorable results for longer periods of time, because the tumor-free period of the patients in this study averaged about nine months. And the theoretically proposed advantages of PBRT, which were the lower occurrence of secondary malignant neoplasm or facial deformity, should be confirmed and bolstered by additional long-term follow-up studies.
REFERENCES
2. Tamboli A, Podgor MJ, Horm JW. The incidence of retinoblastoma in the United States: 1974 through 1985. Arch Ophthalmol 1990;108:128-132.


3. Verhoeff FH. Glioma retinae treated by X-rays, with apparent destruction of the tumor and preservation of normal vision. Trans Am Ophthalmol Soc 1921;19:209-216.


5. Wong FL, Boice JD Jr, Abramson DH, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA 1997;278:1262-1267.


6. Mohney BG, Robertson DM, Schomberg PJ, Hodge DO. Second nonocular tumors in survivors of heritable retinoblastoma and prior radiation therapy. Am J Ophthalmol 1998;126:269-277.


7. Smith LM, Donaldson SS, Egbert PR, et al. Aggressive management of second primary tumors in survivors of hereditary retinoblastoma. Int J Radiat Oncol Biol Phys 1989;17:499-505.


8. Imhof SM, Mourits MP, Hofman P, et al. Quantification of orbital and mid-facial growth retardation after megavoltage external beam irradiation in children with retinoblastoma. Ophthalmology 1996;103:263-268.


9. Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol 1996;114:1339-1343.


10. Shields CL, Shields JA, Needle M, et al. Combined chemoreduction and adjuvant treatment for intraocular retinoblastoma. Ophthalmology 1997;104:2101-2111.


11. Lee CT, Bilton SD, Famiglietti RM, et al. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys 2005;63:362-372.


12. Krengli M, Hug EB, Adams JA, et al. Proton radiation therapy for retinoblastoma: comparison of various intraocular tumor locations and beam arrangements. Int J Radiat Oncol Biol Phys 2005;61:583-593.


13. Munier FL, Verwey J, Pica A, et al. New developments in external beam radiotherapy for retinoblastoma: from lens to normal tissue-sparing techniques. Clin Experiment Ophthalmol 2008;36:78-89.


14. Crowther JG. The cavendish laboratory 1874-1974. 1974. New York: Science History Publication; p. 181.
15. Debus J, Hug EB, Liebsch NJ, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys 1997;39:967-975.


16. Egger E, Schalenbourg A, Zografos L, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys 2001;51:138-147.


17. Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest 1999;116:1313-1319.


18. Croughs P, Deman C, Richard F, et al. Treatment of retinoblastoma using accelerated protons. Bull Soc Belge Ophtalmol 1992;243:81-85.

19. Suit H. The Gray Lecture 2001: coming technical advances in radiation oncology. Int J Radiat Oncol Biol Phys 2002;53:798-809.


20. Gunduz K, Shields CL, Shields JA, et al. The outcome of chemoreduction treatment in patients with Reese-Ellsworth group V retinoblastoma. Arch Ophthalmol 1998;116:1613-1617.


21. Shields CL, Meadows AT, Shields JA, et al. Chemoreduction for retinoblastoma may prevent intracranial neuroblastic malignancy (trilateral retinoblastoma). Arch Ophthalmol 2001;119:1269-1272.


22. Wilson MW, Rodriguez-Galindo C, Haik BG, et al. Multiagent chemotherapy as neoadjuvant treatment for multifocal intraocular retinoblastoma. Ophthalmology 2001;108:2106-2114.


23. Shields CL, Honavar SG, Shields JA, et al. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol 2002;120:460-464.


Fig.┬Ā1
(A) There was no significant change in the tumor size and shape after chemotherapy and brachytherapy. Therefore, we started proton beam radiation therapy (PBRT). (B) Proton dose distribution of an anterior medially oriented oblique beam with the eye rotated in the temporal direction. Radiation was concentrated to the nasal side of the tumor but there was minimal radiation distribution to surrounding normal tissue. (C) Complete regression of the tumor after PBRT.
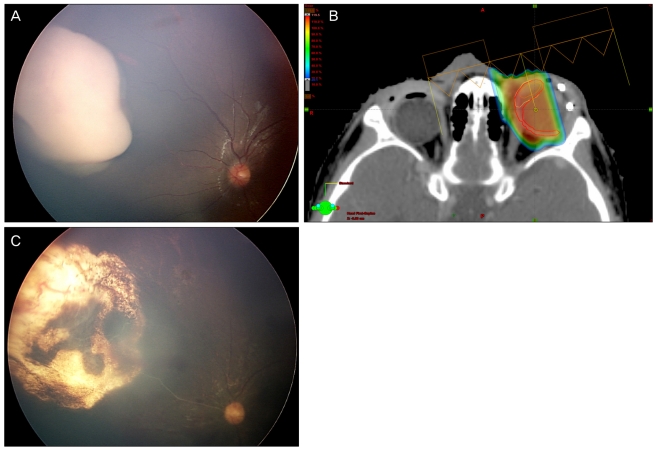
Fig.┬Ā2
(A,B) Subretinal seeding developed after completion of chemotherapy. (C) Proton dose distribution of a right posterior oblique beam with the eye straight position. Radiation covered whole retina behind the lens and there was no radiation distribution to contralateral eye. (D,E) One month after proton beam radiation therapy, there was complete regression of subretinal seeding.
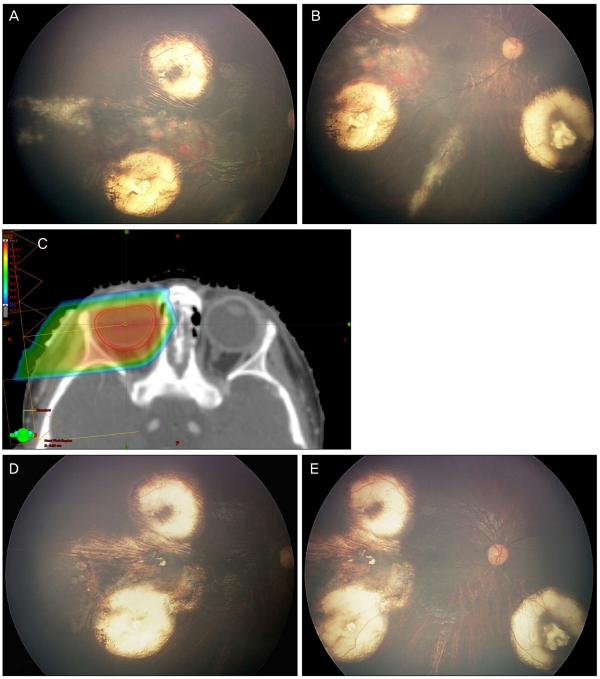
Fig.┬Ā3
(A) There was massive tumor with total retinal detachment and peripheral subretinal seeding at initial presentation. (B) Newly developed diffuse subretinal seeding around the tumor and optic disc during chemotherapy. (C) Proton dose distribution of a lateral beam orientation with the eye in a straight position. The maximum energy of radiation covered the entire retina including proximal optic nerve. (D) There was total regression of subretinal seeding and significant shrinkage of the main tumor after proton beam radiation therapy (PBRT). (E) Three weeks after PBRT, the main tumor increased and multiple tumor reoccurrences developed around the tumor and at the entire ora serrata. We performed enucleation.

- TOOLS
-
METRICS

- Related articles
-
Clinical Result of Prolonged Primary Chemotherapy in Retinoblastoma Patients.2003 June;17(1)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



