Tectonic Deep Anterior Lamellar Keratoplasty in Impending Corneal Perforation Using Cryopreserved Cornea
Article information
Abstract
We report a case of tectonic corneal transplantation for impending corneal perforation to preserve anatomic integrity using cryopreserved donor tissue. An 82-year-old woman exhibiting impending corneal perforation suffered from moderate ocular pain in the left eye for one week. After abnormal tissues around the impending perforation area were carefully peeled away using a Crescent blade and Vannas scissors, the patient received tectonic deep anterior lamellar keratoplasty using a cryopreserved cornea stored in Optisol GS® solution at -70℃ for four weeks. At six months after surgery, the cornea remained transparent and restored the normal corneal thickness. There were no complications such as corneal haze or scars, graft rejection, recurrent corneal ulcer, and postoperative rise of intraocular pressure. Cryopreserved donor lamellar tissue is an effective substitute in emergency tectonic lamellar keratoplasty, such as impending corneal perforation and severe necrotic corneal keratitis.
Corneal perforation, impending perforation, and descemetocele are ophthalmic emergencies that require immediate detection and prompt intervention. Attempts must be undertaken to restore the structural integrity of the cornea as soon as possible using tissue adhesives, conjunctival flap, penetrating keratoplasty, and amniotic membrane transplantation [1]. Tectonic graft is a useful therapeutic option in selected cases of corneal thinning and perforations [2]. The development of the anterior lamellar keratoplasty resulted in deep anterior lamellar keratoplasty (DALK). As such, developing a tectonic DALK is promising a new challenge, as it is not a risk for immune reactions to the endothelium and other intraoperative complications [3,4].
With the advancement in lamellar grafting, the issue of eye bank tissue preservation has become more important. Cryopreservation can be conducted for a long time, and it provides greater availability of tissue for emergencies [5]. In using this storage method, lamellar grafting can have the opportunity to boost corneal transplantation. However, most studies on cryopreservation focused on reducing the endothelial cell damage due to freezing during penetrating keratoplasty [6]. Lyophilized donor tissues have been used in other trials for lamellar keratoplasty [7,8].
Here we report a case of tectonic DALK with the donor cornea preserved at -70℃ in Optisol GS® (Bausch & Lomb, Irvine, CA, USA). Cryopreserved cornea can be a useful tissue in emergency situations for tectonic DALK, such as in impending corneal perforation.
Case Report
An 82-year-old woman experienced an abrupt decrease in visual acuity with associated pain in the left eye for seven days. The visual acuity was FC 30 cm with correction at initial visit. Slit-lamp examination showed a 4 × 4 mm-sized corneal ulceration with severe stromal thinning that bulged anteriorly from the base of the ulceration near the inferior paracentral area (Fig. 1). We used a sterile fluorescein dye to paint the suspected thinning area of perforation, applying gentle pressure from above or below the thinning of the cornea. However, the Seidel test was negative. Anterior chamber depth was maintained deeply and was checked for rare inflammatory cells. There was no visualization of retinal details through the pupil due to severe cortical opacity and nucleosclerosis.
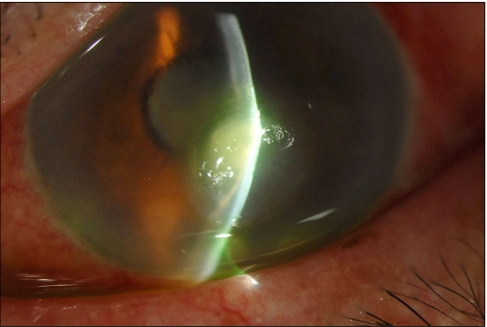
Corneal ulceration with impending corneal perforation was seen by slit lamp examination before tectonic deep anterior lamellar keratoplasty.
A donor cornea was evaluated on the entire globe state. Corneal thickness was measured by pachymetry, while the evaluation of endothelial cell counts and shapes was performed by specular microscopy. A lamellar dissection was created at a thickness of 400 µm and a diameter of 9.0 mm with femtosecond laser (IntraLase™, IntraLase Corporation, Irvine, California, USA). A blunt spatula was used to separate the overlying corneal tissue along the plane of dissection. The anterior lamellar flap was immersed in Optisol GS® solution and was stored at -70℃ in a freezing system, while the posterior lamella flap was used as the Descemet-stripping endothelial keratoplasty in the bullous keratopathy patient. After four weeks of storage, the cryopreserved corneal tissue within a vial was thawed at room temperature.
Afterwards, the epithelium, Bowman's layer, and anterior stromal tissue of the recipient around the impending perforation area were carefully peeled away using a Crescent blade; the irregular margin of the keratectomy bed was trimmed with Vannas scissors. The donor tissue was cut in the same size, attached to the recipient wound, and sutured into the host bed using running 10-0 nylon sutures and two interrupted sutures as an adjustable slip knot (Fig. 2A). The corneal graft was covered with a temporary amniotic membrane patch to reduce the pain and corneal haze (Fig. 2B). Six months after surgery, the cornea remained transparent and approximately 470 µm in the thinnest area (Fig. 2C and 2D). There were no complications, such as suture-related infection, corneal haze or scars, graft rejection, recurrent corneal ulcer, and postoperative rise of intraocular pressure.
Discussion
Moist chamber storage at 4℃ which was first described by Filatov [9] in 1935, is safe for up to 48 hours. The use of tissue culture media such as K-sol®, Corneal storage media®, Dexsol® and Optisol® can prolong the length of storage up to 12 days [10].
Since the successful cryopreservation of spermatozoa in Ringer's solution containing 40% glycerol [11], many human tissues or cells have been preserved via freezing. Cryopreservation of the corneal tissue has a long history; however, most studies have focused on the cryoprotection of endothelial cells. To protect the living endothelial cells from freezing damage, many cryoprotectants such as glycerol [12], dimethyl sulphoxide (DMSO) [13], and dextran [6] were used. Preserving the living endothelial cells for penetrating keratoplasty is essential. The transplantation of cryopreserved human corneas used with cryoprotectants has been limitedly successful in both the short- and long-term [14]. However, with the introduction of short-term storage at 4℃ in Optisol GS® solution, cryopreservation failed to gain wide acceptance owing to its technically demanding nature, costliness, and variable clinical and experimental results achieved [6].
On the other hand, in the field of surgery, penetrating keratoplasty is no longer the gold standard for opaque cornea. The surgical techniques used in lamellar keratoplasty have been improved with DALK and Descemet membrane endothelial keratoplasty [15,16]. Cryopreserved tissues are used for lamellar keratoplasty, and most of them were treated with DMSO or glycerol in the past. Some trials used lyophilized, that is, frozen and dried, tissue [7,8]. With the advance of lamellar keratoplasty, precut buttons using the femtosecond laser and microkeratome, corneal tissue storage techniques and media have increasingly gained much interest. To minimize waste of donor tissue, the preservation and recycling of leftover cornea seem reasonable in countries where there is a shortage in donor cornea tissues.
We preformed tectonic DALK on cryopreserved cornea at -70℃. The cornea was dissected using a femtosecond laser and was dipped in Optisol GS® solution for four weeks. This method does not require an additional special processs, e.g., lyophilization. The benefits of lyophilized tissue are reduced antigenicity and easy distribution [7]. However, the study on epikeratophakia lenticule reveal that neither fresh nor lyophilized tissue elicits immune reactions in non-vascularized corneas of immune recipients or in non-immune recipients [17]. This means that immunogenicity does not matter when the tissue is implanted into the avascular pocket. It is difficult to expect that the keratocytes would survive under the condition of -70℃ without a proper concentration of cryoprotectant. It was reported that the survival rate of keratocytes frozen in preservation media at -40℃ without cryoprotectant were 0 - 0.1% [18]. Even freeze-dried tissue can be used in a lamellar keratoplasty procedure. Only frozen tissue can be used a small area during lamellar keratoplasty. The distribution of the tissue does not matter in countries where there is a shortage in donor tissue, because the ophthalmology department can easily consume cryopreserved lamellar tissue in its own eye bank. Our cryopreserved grafts immersed for four weeks in Optisol GS® solution showed a significant freshly thawed cornea state until the end of surgery. Optisol GS® contains 1% dextran, but it may not be able to protect keratocytes from freezing damage.
The recent report on small-diameter penetrating keratoplasty in eccentric corneal perforation with glycine-cryopreserved tissue seems to be effective [19]. The tectonic DALK, which is used in the case of still-intact Descemet's membrane, does not require endothelial cells. Establishing the safety of DALK for larger grafts in order to use this technique for keratoconus patients is necessary. In this case, we experienced that simple cryopreserved cornea in Optisol GS® solution can be valuable substitutes in emergency tectonic lamellar keratoplasty of a small avascular area, such as in impending perforation. However, more study about the safety regarding the potential structural damage or change would be necessary prior to the recommendation for such use.
Notes
A summary of this paper was presented as a poster at the 101th spring meeting of Korean Ophthalmological Society, April 2009, Daejeon, Korea.
No potential conflict of interest relevant to this article was reported.
