Intraocular Pressure Changes after Vitrectomy with and without Combined Phacoemulsification and Intraocular Lens Implantation
Article information
Abstract
Purpose
To determine sequential intraocular pressure (IOP) changes after pars plana vitrectomy (PPV) with or without combined phacoemulsification and intraocular lens implantation (PE & IOL).
Methods
Consecutive patients who underwent PPV with PE & IOL (combined group) or without PE & IOL (vitrectomy group) were reviewed for postoperative sequential IOPs and the number of IOP lowering medications used. Of the 68 patients (68 eyes) who underwent simple PPV, 41 eyes were allocated to the vitrectomy group, and 27 eyes to the combined group.
Results
The mean IOPs were higher on postoperative days one and two, as compared to preoperative values, in both groups. The mean IOP changes on postoperative day one (10.0 mmHg vs. 5.3 mmHg, p = 0.02) and day two (3.7 mmHg vs. 1.3 mmHg, p = 0.02) were significantly higher in the combined group.
Conclusions
Phacovitrectomy is associated with a higher risk of IOP elevation during the early postoperative period than PPV alone. Caution should be exercised in patients who are vulnerable to IOP fluctuations when combined surgery is indicated.
Cataract surgery combined with pars plana vitrectomy (PPV) has become more common since the development of the phacovitrectomy technique. Significant lens opacities frequently develop after PPV, especially in patients greater than 60 years of age, and some surgeons prefer phacovitrectomy to avoid the need for subsequent cataract surgery [1,2]. The surgeon usually has the option of choosing between phacovitrectomy and PPV alone in patients with vitreoretinal diseases, under the notion that the surgical outcomes are similar. Many reports have shown that combined cataract extraction and PPV is safe and effective, providing more rapid visual rehabilitation and having a similar complication rate to that observed in sequential surgery [3,4].
Postoperative intraocular pressure (IOP) elevation is a frequently encountered complication after PPV. Substantial IOP increase after PPV alone is found in up to 40% of patients within 48 hours [5,6]. Around 15% to 56% of patients develop a transient IOP elevation within a few days after combined PPV and cataract extraction [3-11].
As in many other procedures combined with PPV, combined phacoemulsification and intraocular lens implantation (PE & IOL) may increase the risk for additional IOP elevation in the early postoperative period. Conversely, the long-term IOP lowering effect of cataract surgery may lead to decreased IOP in the late postoperative period [12]. We were unable to find any reports comparing postoperative IOP changes after PPV alone versus PPV with PE & IOL through a comprehensive Medline literature search. Therefore, we performed this study to evaluate sequential IOP changes after PPV with and without combined PE & IOL and to determine whether combined PE & IOL has any significant effect on early or late postoperative IOP changes after PPV.
Materials and Methods
The medical records of 68 patients who underwent PPV with and without cataract extraction were retrospectively reviewed. Forty-one eyes of 41 patients who underwent PPV alone were allocated to the vitrectomy group, and 27 eyes of 27 patients who underwent PPV with PE & IOL were allocated to the combined group. All surgeries were performed by a single, experienced vitreoretinal and cataract surgeon (KHP) between October 1, 2005 and October 30, 2006 at Seoul National University Bundang Hospital. Patients who underwent uncomplicated cataract surgery with PPV and who were followed for more than three months were enrolled in the study. Patients who underwent simple PPV were also enrolled. Patients with a history of any of the following conditions were excluded from the study: 1) underlying glaucoma, 2) high myopia, 3) prior intraocular surgery, 4) penetration injury of the eyeball, 5) severe proliferative vitreoretinopathy, 6) severe tractional retinal detachment, 7) history of previous uveitis, 8) posterior capsule rupture during phacoemulsification, 9) intraoperative procedure, such as silicone oil injection, cryotherapy, scleral buckling, intravitreal gas injection, intravitreal triamcinolone injection, or 10) endolaser photocoagulation of more than two quadrants of the retina [5,13]. Preoperative data obtained from electronic medical charts included patient age, gender, operative eye, indication for surgery, history of previous ocular surgery, IOP, anterior segment findings, and posterior segment findings. Thorough preoperative examinations were performed one to two days before surgery and included IOP measurement with a Goldmann applanation tonometer.
In all cases, a traditional 20-gauge standard three-port PPV was performed. In the combined group, PE & IOL was performed before PPV through a 2.75 mm superior clear corneal incision. A 10-0 nylon suture was applied to the corneal wound if there was any wound leakage. The same ocular viscoelastic device, Healon GV (sodium hyaluronate 1.4%), was used in all cases.
At each postoperative period, IOP, the number of glaucoma medications, complications, such as anterior chamber inflammation, angle closure, or synechiae, hyphema, vitreous hemorrhage, and secondary glaucoma status were recorded. Topical antibiotics and topical fluorometholone acetate (0.1%) were routinely used for one month.
Statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). The paired t-test and Wilcoxon signed rank test were used to analyze postoperative IOP changes and the number of glaucoma medications, as compared to preoperative data. The IOP ratio (postoperative IOP/preoperative IOP) was compared between the groups at each point using the independent t-test and Mann Whitney U-test. A p-value of < 0.05 was considered to be statistically significant.
Results
The patients' preoperative data are shown in Table 1. Preoperative patient characteristics, including age, gender, and ratios of proliferative diabetic retinopathy were not significantly different between the two groups, but the preoperative IOP was lower in the combined group (Table 1). The indications for PPV included complications from proliferative diabetic retinopathy (51.5%), such as vitreous hemorrhage and tractional retinal detachment, macular pucker (19.1%), and vitreous hemorrhage of non-diabetic etiology (16.2%), which were not significantly different between the two groups (Table 1). Indications for combined cataract surgery included a posterior subcapsular lens opacity score ≥ 3, nuclear opalescence/color score ≥ 4, or cortical score ≥ 3, as measured by the Lens Opacity Classification System III [14]. The IOL was implanted in the bag in all cases.
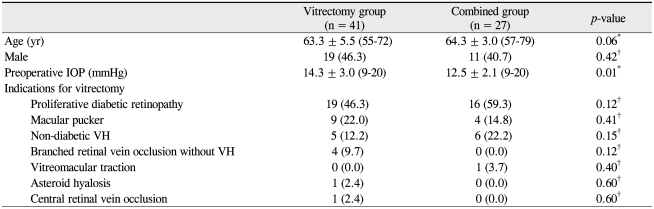
Clinical characteristics of the patients who underwent vitrectomy with (combined group) or without cataract surgery (vitrectomy group)
Postoperative IOP change in the vitrectomy group, showed a significant IOP increase from 14.3 ± 3.0 mmHg (preoperative) to 19.6 ± 7.2 mmHg on postoperative day one (p < 0.01), and 15.5 ± 3.5 mmHg on day two (p = 0.03, paired t-test). The IOP was similar to the preoperative level at postoperative day three, week one, and week three. The IOP was actually lower than preoperative values at one, two, and three months postoperatively. In the combined group, a significant IOP increase occurred from 12.5 ± 2.1 mmHg (preoperative) to 22.4 ± 8.2 mmHg on postoperative day one (p < 0.01), 16.2 ± 4.5 mmHg on day two (p < 0.01), and 14.4 ± 3.0 mmHg on day three (p < 0.01). The IOP returned to the preoperative level at postoperative weeks one and three. The IOP was significantly lower than the preoperative values at months one, two, and three (Table 2 and Fig. 1).
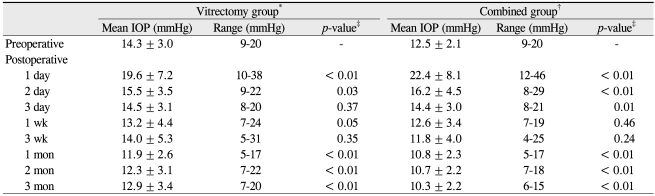
The sequential changes of intraocular pressure (IOP) after surgery in the vitrectomy group and the combined group

Sequential changes of postoperative intraocular pressure (IOP) after surgery in the vitrectomy group and combined group. Compared to the preoperative IOP values, postoperative IOP showed significant difference in early and late postoperative periods in the vitrectomy group and combined group. *p<0.05 by paired t-test in the vitrectomy group; †p<0.05 by paired t-test in the combined group.
IOP spikes (≥ 30 mmHg) occurred on postoperative day one in 9.8% (4/41) of patients in the vitrectomy group and in 14.8% (4/27) of patients in the combined group; there was no statistically significant difference between the two groups (p = 0.70, Fisher's exact test). The highest IOP recorded on postoperative day one was 36 mmHg in the vitrectomy group and 48 mmHg in the combined group. These patients showed severe inflammation in the anterior chamber, yet angles were widely open and were free of synechia. IOP lowering medications were prescribed for these patients, and none of the eyes showed IOP ≥ 30 mmHg after the second postoperative day.
The mean IOP change in the combined group was significantly higher than that in the vitrectomy group on postoperative days one (p = 0.02) and two (p = 0.02). The mean IOP changes were not significantly different between the two groups at any other timepoint (Table 3).
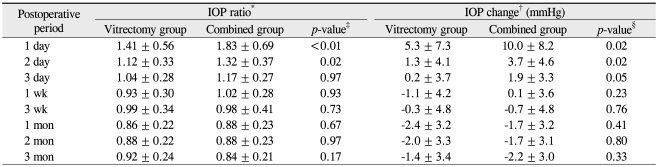
Comparison of mean intraocular pressure (IOP) ratio and IOP change (mmHg) between the vitrectomy group and the combined group after surgery
The IOP ratio was calculated at each postoperative time point, and the logarithmic value was compared between the two groups. The IOP ratios in the vitrectomy and combined groups were 1.41 ± 0.56 and 1.83 ± 0.69, respectively, on postoperative day one (p < 0.01); and 1.12 ± 0.33 and 1.32 ± 0.37, respectively, on postoperative day two (p = 0.02, independent t-test). The IOP ratio was significantly higher in the combined group up to day two. The IOP ratios were not significantly different between the two groups at any other timepoint (Table 3 and Fig. 2).
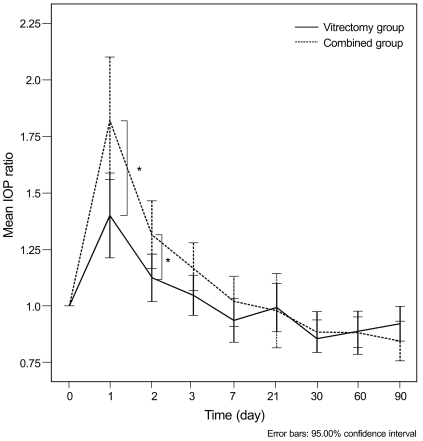
Comparison of the sequential changes of intraocular pressure (IOP) ratio between the vitrectomy group and combined group. The IOP ratios (postoperative IOP/preoperative IOP) were significantly higher in the combined group on postoperative days one and two. *p<0.05 by independent t-test.
There was no significant difference between the two groups with regard to the mean number of medications in use at any follow-up timepoint (p = 0.12-0.96). Transient postoperative IOP elevation was well controlled with the use of glaucoma medications, and the mean number or glaucoma medications was less than 0.1 after the first postoperative week in both groups.
The risk factors associated with IOP increase ≥ 10 mmHg on day one after PPV were estimated using univariate and multivariate analyses (Table 4). Diabetic retinopathy (p = 0.03) and combined cataract extraction (p = 0.01) were significant risk factors by univariate analysis. However, multivariate analysis showed that combined cataract extraction was the only significant risk factor (p = 0.04, linear logistic regression analysis). Stratified analysis controlling for diabetes also showed a significantly larger number of patients with IOP elevation on postoperative day one in the combined group (p = 0.03, Mantel-Haenszel common odds ratio).
Discussion
The major objectives of this study were to investigate the sequential IOP change after PPV and to determine the risk of combined PE & IOL on IOP elevation. In this study, an immediate mean IOP rise was found up to two or three days after PPV. This transient IOP elevation following PPV, whether clinically significant or not, has been explained by open or closed angle mechanisms, including angiogenic, inflammatory, or blood-mediated factors [5,13]. A recent ultrasound biomicroscopy (UBM) study by Lee et al. [15] reported a 42% increase in ciliary body thickness and a decrease in anterior chamber depth after simple PPV, which persisted five days postoperatively. This time interval of ciliary body change was similar to the period of transient IOP elevation seen in our study.
According to our results, combined PE & IOL seems to exert an additive effect on transient IOP elevation after PPV, up to postoperative day two. It may be postulated that more inflammation of the trabecular meshwork was induced by cataract surgery, leading to additional IOP elevation in the first few days after surgery. Combined cataract surgery may also cause anterior segment inflammation with disruption of the blood-aqueous barrier [16,17]. Unfortunately, we did not perform UBM of the ciliary body, and the precise mechanism of IOP change is unknown. Postoperative remnant materials in the anterior chamber are other potential causes of immediate IOP rise after cataract extraction. However, meticulous irrigation and aspiration were performed during cataract surgery to remove the viscoelastics and cortex; therefore, these factors are less likely to have contributed to postoperative IOP elevation.
Comparing with cataract surgery alone, previous studies agree that cataract surgery decreases IOP in the first few days after surgery in patients without underlying glaucoma [18-20]. Shingleton et al. [18] demonstrated an immediate IOP rise in the first few hours after cataract surgery, with 10% of patients showing an IOP spike of ≥ 35 mmHg IOP. However, a continuous decline of IOP was observed after this peak, and the mean IOP on postoperative day one decreased up to 5 mmHg [18-20]. In contrast, there are few studies concerning sequential IOP changes after PPV. Desai et al. [5] found that 40% of patients had IOP spikes requiring medications; however, IOP reached baseline within 24 hours. In our study, an IOP increase of 5 mmHg to 10 mmHg was observed on day one after PPV or phacovitrectomy. Although direct comparison is not possible due to different underlying conditions or surgical procedures, postoperative IOP seems to decline more rapidly after cataract surgery, while PPV or phacovitrectomy seems to increase IOP on the first couple of days before showing a decline [5,18-20].
With regard to IOP spikes (≥ 30 mmHg), 10% to 15% of patients in both groups showed an IOP spikes after PPV on postoperative day one. These patients were associated with excessive inflammation and fibrin membranes in the anterior chamber greater than grade 3 and had an underlying retinal vascular disease of branched retinal vein occlusion or proliferative diabetic retinopathy. Fibrin membranes in the anterior chamber and proliferative diabetic retinopathy are both identified to be risk factors of IOP elevation after intraocular surgery [4,5]. In our study, prompt initiation of IOP lowering medication restored the IOP levels to < 30 mmHg by the second postoperative day in all patients. Therefore, the clinical significance of transient postoperative inflammation and IOP spikes may be limited to those who are vulnerable to IOP fluctuation and who have low thresholds to optic nerve damage.
The late postoperative IOP lowering effect after PPV is another interesting finding of this study. A significant decline of mean IOP after PPV was observed from the first postoperative month up to the end of follow-up in both groups. This late postoperative IOP lowering effect has been described after simple cataract surgeries, but not after PPV or combined phacovitrectomy [12,18-22]. Instead, late postoperative glaucoma has been reported in up to 26% of patients after PPV [13,23]. The difference between studies may be due to the presence of risk factors of IOP elevation in previous studies, e.g., a greater proportion of severe diabetic retinopathy or complicated PPV, including combined procedures like scleral buckling and tamponade with gas or silicone oil, etc. In the current study, only simple PPV cases were included, and we found no severe anterior chamber inflammation or late neovascular glaucoma in any of our patients, even in those with proliferative diabetic retinopathy.
The exact mechanism of long-term IOP reduction after PPV is unknown. Some reports have evaluated the ciliary body and angle opening with UBM after PPV, but there was no evident change after two months [15]. There is still the possibility of subtle structural change that might not have been detected with the UBM. Further investigations are required to reveal the long-term changes relating to increased outflow facility after PPV. On the other hand, the long-term IOP reduction after cataract surgery has been explained by the improvement of outflow facility, deepening of the anterior chamber and posteriorization of the lens-iris diaphragm after cataract surgery [19,24,25]. These factors may also contribute after PPV, although the exact mechanism remains to be elucidated.
This study is limited by its retrospective nature and small number of cases. A relatively small number of patients were enrolled, because all possible confounding factors that might have influenced postoperative IOP changes were excluded. Secondly, the preoperative mean IOPs differed between the two groups. However, the mean baseline IOPs were both in the low teens (11-15 mmHg), and IOP ratios were compared to adjust for the preoperative difference. Finally, since the absolute values of IOP rise were relatively small, the clinical significance of such mild early postoperative IOP elevation may be argued. However, such rise may be critical to patients who are vulnerable to IOP fluctuations which must not be overlooked. Prophylactic glaucoma medications are known to be beneficial in preventing acute postoperative IOP spikes after cataract surgery in such patients [26,27].
In conclusion, combined PE & IOL and PPV are associated with a higher risk of IOP elevation than PPV alone during the early postoperative period. Fortunately, most of the pressure elevations are clinically insignificant or transient, which could be controlled medically. Nevertheless, caution should be exercised in patients whose optic nerves and retinal perfusion are vulnerable to IOP fluctuations, especially when combined surgery is indicated.
Notes
This article was presented at the Association for Research in Vision and Ophthalmology 2007 annual meeting, Fort Lauderdale, FL, USA, May 2007.
No potential conflict of interest relevant to this article was reported.
