A Novel Therapeutic Target in Inflammatory Uveitis: Transglutaminase 2 Inhibitor
Article information
Abstract
Purpose
Our goal was to investigate the effects of inhibition of transglutaminase 2 (TGase 2) on endotoxin-induced uveitis (EIU)
Methods
EIU was induced in female Lewis rats by single footpad injections of 200 µg of lipopolysaccharide (LPS). TGase 2 inhibitors were administered intraperitoneally 30 minutes before and at the time of LPS administration. Rats were sacrificed 24 hours after injection, and the effects of the TGase 2 inhibitors were evaluated by the number of intraocular inflammatory cells present on histologic sections and by measuring the TGase 2 activity and TGase products in the aqueous humor (AqH). TGase 2 substrates were also assayed in AqH from uveitis patients.
Results
Clinical indications of EIU, the number of cells present on histologic sections, and TGase 2 activity in AqH increased in a time-dependent manner, peaking 24 hours after LPS injection. Inflammation in EIU was significantly reversed by treatment with TGase inhibitors. A 23-kDa cross-linked TGase substrate was identified in the AqH from EIU rats and uveitis patients. MALDI-TOF analysis showed that this substrate in uveitis patients was human Ig kappa chain C region.
Conclusions
TGase 2 activity and its catalytic product were increased in the AqH of EIU rats. TGase 2 inhibition attenuated the degree of inflammation in EIU. Safe and stable TGase inhibitors may have great potential for the treatment of inflammatory uveitis.
Acute anterior uveitis is an inflammatory disorder that involves the iris and parts of the ciliary body. Endotoxin-induced uveitis (EIU), an animal model similar to acute ocular inflammation in humans [1], is characterized by breakdown of the blood-aqueous barrier, leading to an extravasation of vascular fluid protein and massive infiltration of inflammatory cells, predominantly neutrophils, macrophages and T lymphocytes, into the anterior chamber of the eye [2, 3]. Although corticosteroids are effective in reducing inflammation, they are associated with a wide range of complications, including cataract, increases in intraocular pressure, and increased susceptibility to microbial infection, if administered over extended time periods [4]. In addition, some patients are resistant to corticosteroids. Conventional steroid-sparing drugs, including antimetabolites, alkylating agents, T-cell inhibitors, and antibodies, have provided significant advances in disease control [5, 6]. However, these medications may also have serious side-effects, requiring regular monitoring of patients.
Transglutaminase 2 (TGase 2, EC2.3.2.13) is a Ca2+-dependent enzyme that catalyzes the formation of isopeptide linkages between the carboxamide groups of protein-bound glutamine residues and the ε-amino groups of protein-bound lysine residues [6, 7]. TGase 2 is expressed at low levels in many different tissues but is inappropriately activated in a variety of pathological conditions, including neurodegenerative diseases, atherosclerosis, inflammatory diseases, autoimmune diseases, and fibrosis [8]. In many inflammatory diseases, including celiac disease, Crohn's disease, and sporadic inclusion-body myositis, increased TGase activity is closely associated with inflammation [9, 10]. Moreover, the inhibition of TGase 2 may reverse the inflammatory process in brain injury, allergic conjunctivitis, and in a lung fibrosis model [11-13].
We found that TGase 2 expression is dramatically increased in an animal model of allergic conjunctivitis and that rationally designed TGase inhibitors reversed the inflammatory process in this model [14]. The association between increased TGase 2 activity and uveitis suggests that TGase 2 expression may play a significant role in EIU pathogenesis. We therefore assessed whether TGase 2 expression is increased during the progression of EIU and if this process can be reversed by TGase inhibitors.
Materials and Methods
Animals and EIU
Uveitis was induced in 8-10 weeks old female Lewis rats weighing 190 g to 210 g by a 200 µg injection of lipopolysaccharide (LPS; Salmonella typhimurium; Sigma-Aldrich, San Francisco, CA, USA) dissolved in sterile, pyrogen-free saline into the foot. Clinical signs of uveitis were monitored by direct slit-lamp biomicroscopy of the anterior eye segment (Table 1) [15].
Histologic evaluation
Rats were euthanized every 4 hours after LPS injection, up to 48 hours. The eyes were enucleated immediately and stored in a mixture of 10% formalin and 2.5% glutaraldehyde for 24 hours, then embedded in paraffin. Sagittal sections (5 µm thick) were cut near the optic nerve head and stained with hematoxylin and eosin. The number of infiltrating cells was counted on each histologic section of the iris-ciliary body. Neutrophils were counted by an investigator unaware of the treatment groups. For each animal, the number of cells represented the mean count in three serial sections.
TGase 2 activity in EIU
Aqueous humor (AqH) was collected immediately after treatment by anterior chamber puncture (15-20 µL/rat) using a 30-gauge needle at 0, 4, 8, 12, 24, 32, 40, and 48 hours after LPS injection. TGase activity in AqH was assayed by measuring the covalent binding of [1,4-14C] putrescine to succinylated casein [6]. Each AqH sample was incubated for 1 hour at 37℃ with 0.5 mL reaction mixture containing 0.1 M Tris-acetate (pH7.5), 1% (wt/vol) succinylated casein, 1 mM EDTA, 10 mM CaCl2, 0.5% (wt/vol) lubrol PX, 5 mM DDT, 0.15 M NaCl and 0.5 mCi of [1,4-14C] putrescine dihydrochloride (DuPont-New England Nuclear, Boston, MA, USA). The reaction was terminated by the addition of 4.5 mL of cold (4℃) 7.5% (wt/vol) trichloroacetic acid (TCA). The TCA-insoluble precipitates were collected onto GF/A (Millipore, Bedford, MA, USA) glass fiber filters, washed with cold 5% (wt/vol) TCA, dried and counted. The negative control was TGase preincubated with buffer only.
Western blot analysis (cross linking)
AqH samples were electrophoresed on 10-20% gradient SDS gels in Tricine buffer (Invitrogen, Carlsbad, CA, USA) and transferred to nitrocellulose filters, which were incubated with 5 µg/mL mouse monoclonal anti-isopeptid antibody, followed by 0.1 µg/mL anti-mouse, 814-MAM (CovalAb, Lyon, France) secondary antibody. The blots were developed by enhanced chemiluminescence (Pierce, Milwaukee, WI, USA).
TGase inhibitors
The TGase inhibitor, R2 peptide (KVLDGQDP), which has been shown to be effective both in vitro and in vivo, was synthesized as described previously [5]. Cystamine (CTM), which inhibits TGase activity by blocking the access of the glutamate residue in substrate proteins from the TGase active site, was obtained from Sigma-Aldrich [16]. Thirty minutes before and immediately after LPS injection, the rats were injected intraperitoneally with R2 (10 mg/kg, 500 µm), CTM (6.0 mg/kg, 500 µm), or dexamethasone (2 mg /kg) in 0.2 mL pyrogen-free saline. Control rats were injected with 0.2 mL pyrogen-free saline 30 min before and immediately after LPS injection. AqH samples were collected 24 hours after LPS injection.
Human AqH
We obtained 200 µL AqH aliquots from human eyes with uveitis using 30-gauge needles. All procedures adhered to the tenets of the Declaration of Helsinki, and local approval was received from the Investigational Review Board of the Asan medical center. Informed consent was obtained from each patient and control.
1-DE and In-gel tryptic digestion
Protein samples (10 µg) were diluted in 4X NuPAGE LDS sample buffer (Invitrogen), separated by SDS-PAGE using 4-12% NuPAGE Bis-Tris Gel (Invitrogen), and visualized by staining with Coomassie blue. Protein spots excised from the gel were washed with 50 mM ammonium bicarbonate (ABC) for 10 minutes and with 50 mM ABC/70% acetonitrile for 10 minutes repeatedly until the blue color disappeared. Each de-stained gel piece was incubated with 50 µLof 10 mM dithiothreitol at 55℃ for 20 minutes, washed with 50 mM ABC/70% ACN for 10 minutes, incubated with 50 µL of 10 mg/mL iodoacetamide in the dark for 20 minutes, and again washed with 50 mM ABC/70% ACN for 10 minutes. Each gel piece was dried and incubated overnight with 10 ng of modified trypsin (Promega) in 20 µl of 50 mM ABC at 37℃. The supernatant was collected after digestion, and the gel piece was extracted with 100 µl of 0.1% trifluoroacetic acid (TFA). The extracts were combined, dried in a SpeedVac (Labconco, Fort Scott, KS, USA) and resuspended in 0.1% TFA for immediate mass spectrometric analysis or stored at -20℃ until use.
MALDI-TOF MS analysis
Peptide and fragment masses were determined using MALDI-TOF mass spectrometry (4700 Proteomics Analyzer; Applied Biosystems, Framingham, MA, USA). The matrix of choice was 5 mg/mL-α-cyano-4-hydroxycinnamic acid (CHCA; Sigma, St. Louis, MO, USA) in 0.1% aqueous TFA/ACN (1:1, v/v). The samples were desalted using Zip-Tips C18 (Millipore), eluted in matrix solution directly on the target, then air-dried. The analyses were performed in positive ion reflectron mode, and spectra were calibrated at 50 ppm tolerance using the [M+H] ion from 4700 Cal Mix (Applied Biosystems); des-Arg1-bradykinin (Mr 904.4681), angiotensin 1 (Mr 1296.6853), Glu-fibrinopeptide B (Mr 1570.6774), ACTH 1-17 clip (Mr 2093.0867), ACTH 18-39 clip (Mr 2465.1989), and ACTH 7-38 clip (Mr 3657.9294). For MS/MS experiments, the collision energy, which is defined by the potential difference between the source acceleration voltage (8 kV) and the floating collision cell (7 kV), was set at 1 kV, and calibration was performed at 0.1 Da tolerance with Glu-fibrinopeptide B peptide. Inside the collision cell, the selected ions collided with air at a pressure of 2×10-6 Torr. The MS/MS spectra of selected ions were the summation of 30 sub-spectra, each acquired with 50 laser pulses. Data analysis and MS/MS database searching was performed using Data Explorer (Applied Biosystems, Foster, CA, USA) and MASCOT (Matrix Science Ltd., London, UK).
Results
TGase 2 activity in EIU
When we assayed the time course of EIU, we found that the peak of inflammation, as shown by biomicroscopy, was detected 24 hours after LPS stimulation (Fig. 1A). At this time, hyperemic irises and miotic pupils with or without hypopyon were prominent. The numbers of inflammatory cells on the histologic sections were significantly increased at 24 and 30 hours after LPS stimulation and decreased thereafter (Fig. 1B). Total TGase 2 activity in the AqH was increased at 24 and 30 hours after LPS stimulation (Fig. 1C), in parallel with the severity of inflammation.

Disease activity and increasing transglutaminase 2 (TGase 2) activity over time in endotoxin-induced uveitis (EIU). EIU as induced in female Lewis rats by a single foot pad injection of 200 µg lipopolysaccharide (LPS), and disease activity and TGase 2 activity in aqueous humor (AqH) were determined every 4 hour after LPS administration. (A) Clinical findings were assessed on a scale of 0 to 4 according to the extent of inflammation and tissue damage. Peak inflammation was seen 24 hr after LPS stimulation (mean±SD of 10 eyes). (B) Cellular infiltration over time. Infiltration peaked 24 to 28 hr after LPS stimulation (mean±SD of 10 eyes). (C) TGase 2 activity over time. TGase activity increased 130-fold 24 hr after LPS stimulation (mean± SD, n=3). (D) Western blotting using a TGase cross-linked antibody, revealing that a 23-kDa protein increased concomitantly with increases in TGase 2 activity.
TGase 2 substrates in EIU
To test whether TGase 2 substrates were present in the AqH in EIU, we used Western blotting to assay for TGase catalytic products. Using antibodies crosslinked to TGase, we detected a 23 kDa protein (Fig. 1D), the amount of which increased concomitantly with the increase in TGase 2 activity (Fig. 1C).
Effect of TGase inhibitors in EIU
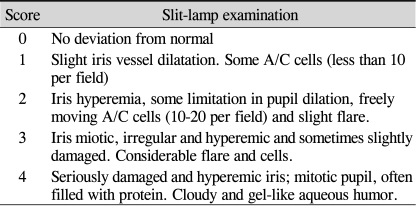
Severe inflammation was observed in the anterior segment of the eyes of rats with EIU 24 hours after LPS administration. Treatment with the TGase inhibitor R2 (KVLDGQDP) reduced inflammation two-fold (Fig. 2A), as well as reducing the number of infiltrating cells on histologic sections by three-fold (Fig. 2B). In contrast, CTM did not show anti-inflammatory effects on EIU (Fig. 2C).
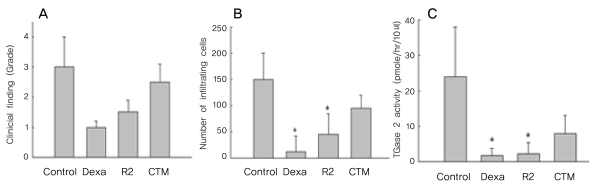
Effect of transglutaminase (TGase) inhibitors on disease activity and TGase 2 activity in Endotoxin-induced uveitis Rats were injected intraperitoneally with R2 peptide (KVLDGQDP,10 mg/kg, 500 µm), cystamine (CTM, 6.0 mg/kg, 500 µm) or dexamethasone (2 mg/kg) 30 minutes before and at the time of lipopolysaccharide administration. Disease activity and TGase 2 activity in aqueous humor were determined 24 hr later. (A) Grading of inflammation, showing a two-fold reduction in the R2-treated rats (mean±SD of 6-8 eyes). (B) Number of infiltrating cells on histologic sections, showing a three-fold reduction in the R2-treated group (mean±SD of 6-8 eyes). (C) TGase 2 activity, showing a ten-fold reduction in the R2 treated rats (mean±SD of 6-8 eyes). *Significantly different from control. p<0.001 by unpaired Student t-test.
Identification of TGase 2 substrate
In addition to the finding that a 23 kDa protein bound by anti-TGase 2 antibodies in AqH increased significantly at 24 and 30 hours after LPS injection but decreased thereafter, we observed increased amounts of a 23 kDa cross-linked molecule in human AqH from eyes with uveitis (Fig. 3A). To identify the protein, this band was subjected to MALDI-TOF analysis. We found that this band was compatible with the human (P01834) Ig kappa chain C region (Fig. 3B-3E).
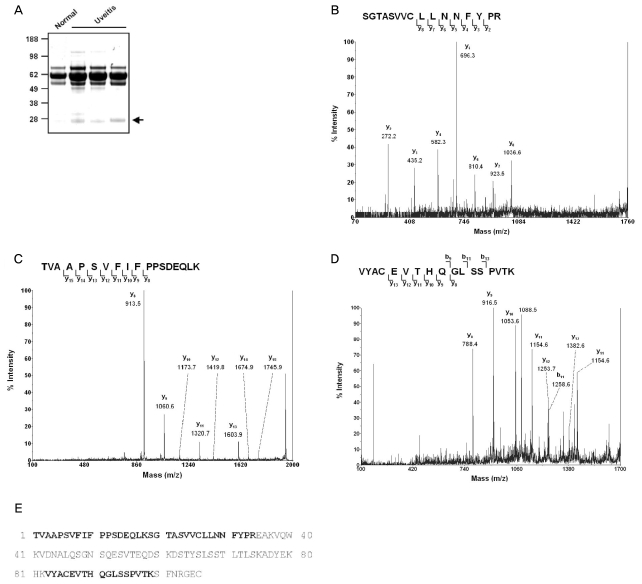
MS/MS identification of transglutaminase 2 (TGase 2) substrate in Aqueous humor (AqH) from uveitis patients.
(A) AqH samples from the eyes of human patients with uveitis were obtained using a 30-gauge needle. Protein samples (10 g) were resolved in 4-12% gels and stained with Coomassie blue. The indicated bands were excised for in-gel tryptic digestion. (B) MS/MS spectra of 1797.9 peptide. (C) MS/MS spectra of 1946.1 peptide. (D) MS/MS spectra of 1876.0 peptide. (E) Amino acid sequence of Ig chain C region. The identified peptides are indicated in bold.
Discussion
We showed that increased total TGase 2 activity in EIU correlates with clinical findings and with increases in inflammatory cell numbers. These results were not surprising because TGase 2 has been shown to be a marker of inflammation [17]. However, our finding that TGase 2 activity corresponded to the degree of inflammation in EIU in a time-dependent manner suggests that TGase 2 may play a key role in the inflammatory process in EIU. TGase 2 expression has been shown to increase in other inflammatory diseases, including inflammatory myositis, LPS-induced inflammation, and celiac disease [10, 12, 15, 16]. Moreover, TGase 2 was recently reported to activate NF-kB, which is responsible for the generation of the proinflammatory mediators nitric oxide and TNF-α [13, 17]. Increased expression of TGase 2 may exacerbate inflammation by amplifying NF-kB activation through I-kBa depletion. Furthermore, TGase 2 inhibition reduced nitric oxide generation during LPS-induced inflammation [13, 18].
When we tested the effects of R2, a peptide inhibitor of TGase, on inflammation in EIU, we found that this inhibitor reduced inflammation, although to a smaller extent than what has been seen with steroids. In contrast, CTM did not have beneficial anti-inflammatory effects, possibly due to its poor ocular permeability. The ability of R2 to gain access to the eye may also be limited owing to its pure octapeptide structure, which may result in its anti-inflammatory effect being lesser than that of steroids [19, 20]. Efforts are needed to develop safe, specific inhibitors of TGase 2 that are able to effectively permeate the eye. Also, the anti-inflammatory effect of TGase inhibitors was maintained while there was active inflammation on EIU (data not shown), but as the LPS-induced inflammatory response in EIU is naturally diminished after 48 hours, we were unable to determine the duration of the anti-inflammatory effect of the TGase inhibitors.
We found that a 23 kDa cross-linked molecule in AqH increased in concentration in parallel with TGase 2 activity and the inflammatory process in EIU. Interestingly, increased quantities of a protein band of the same size were also observed in human AqH from uveitis patients. MALDI-TOF analysis of this molecule showed that it is a human (P01834) Ig kappa chain C region, suggesting that TGase 2 may aggregate with Ig kappa chains, and that these aggregated molecules may play a role in inflammatory uveitis. Plasma cells usually produce excess amounts of light chains relative to heavy chains and secrete free light chains [21]. Under normal circumstances this does not pose a problem, however occasionally free monoclonal light chains aggregate in various pathological forms. Severe light chain aggregation has been observed in light chain amyloidosis as well as in light chain deposition disease. The Km allele of Ig is frequently associated with anterior uveitis, and immune complexes have been detected in the AqH of eyes with endogenous uveitis [21]. Our results suggest that light chain structures may be changed by cross-linking to TGase 2, and that this change may lead to the inflammatory responses seen in EIU.
In summary, we demonstrated that TGase 2 is actively involved in the inflammatory process of EIU, and that this process was effectively reversed by the TGase2 inhibitor, R2. TGase 2 may be a potential novel therapeutic target in autoimmune uveitis.
Acknowledgements
This study was supported by a research grant (#2004-357) from Institute for Life Sciences, Seoul, Korea.