|
 |
| Korean J Ophthalmol > Volume 23(3); 2009 > Article |
Abstract
Purpose
To study the concentration of vascular endothelial growth factor (VEGF) in the aqueous humor before and after intracameral injection of bevacizumab in eyes with neovascular glaucoma, and to detect the duration of an anti-VEGF effect of bevacizumab in the anterior chamber.
Methods
In this prospective interventional case series, 1.25 mg of bevacizumab was injected into the anterior chamber of five eyes in five neovascular glaucoma patients. Aqueous humor samples were obtained just before intracameral injection of bevacizumab and two weeks after injection. The concentrations of VEGF in the aqueous humor were measured using ELISA. To investigate corneal endothelial damage after intrecameral bevacizumab injection, specular microscopy was performed before injection and two weeks after injection. Slit lamp photo and iris fluorescent angiography was performed to determine the regression of iris neovascularization.
Results
After injection, substantial regression of neovascularization or fluorescein leakage was seen in all treated eyes. The VEGF concentrations in the aqueous humor in eyes with NVG were 1181.8±1248.3 pg/mL before intracameral injection of bevacizumab. Two weeks after injection, the VEGF concentrations decreased to 33.2±12.2 pg/mL (p=0.04, Wilcoxon signed rank test). There were no significant changes in IOP or corneal endothelial cells.
Vascular endothelial growth factor (VEGF) is akey regulator of pathological ocular neovascularization and is elevated in the aqueous humor of patients with neovascular glaucoma (NVG) secondary to proliferative vasculopathies such as proliferative diabetic retinopathy (PDR) and central retinal vein occlusion (CRVO).1,2
In addition to VEGF, other substances that may have a role in the development of NVG include basic fibroblast growth factor, platelet-derived growth factor, and insulin-like growth factor-I.3-7
Preparations that inhibit the effects of VEGF have recently become available.
Among these, bevacizumab (Avastin; Genentech, San Francisco, CA, USA), a recombinant humanized monoclonal immunoglobulin antibody, is an anti-human VEGF agent approved as an adjunct treatment for colorectal cancer.8 Its off-label intravitreal use has shown promise for treatment of neovascular age-related macular degeneration, proliferative diabetic retinopathy and macular edema secondary to central retinal vein occlusion.
Intracameral injection of bevacizumab is currently in clinical trials, and some studies have shown that intracameral as well as intravitreal injection of bevacizumab result in a remarkable reduction in aqueous humor levels of VEGF and iris neovasculization.2 But there is little information about the proper timing for subsequent injections of intracameral bevacizuamb, or about the short term side effects of intracameral bevacizumab to the cornea. In this study, we injected bevacizumab into the anterior chambers of NVG patients, whose neovascularization is secondary to PDR or CRVO, and compared the levels of VEGF in the aqueous humor before injection and two weeks after injection. We also investigated the toxic effectsof bevacizumab on corneal endothelial cells using specular microscopy.
After obtaining informed consent, we collected operating room samples of aqueous humor from five human subjects (age range, 43-87 years; mean, 64.2+13.1). Clinically, three of the patients had a proliferative diabetic retinopathy and two had a CRVO. All patients suffered from neovascular glaucoma due to retinal ischemia. Complete sessions of laser photocoagulation were performed on the ischemic retinas of all patients and bevacizumab was injected intravitreally on three of five eyes during the outpatient follow up period, but no therapeutic intervention on the retina was done within six months before and two weeks after first intracameral bevacizumab injection. On gonioscopic examination, three patients had peripheral anterior synechia of about 120 degrees and angles were partially opened. Another two patients had peripheral anterior synechia around 360 degrees with closed angle. The mean intraocular pressure (IOP) was 29.2±10 mmHg (16 to 44 mmHg). All of the patients had used two to three anti-glaucoma drugs to lower intraocular pressure and maintained use of these medications after intracameral bevacizumab injection.
Aqueous humor was also sampled before cataract surgery from eight eyes in eight patients (age range, 70-86 years mean, 67.4+10.8) with cataracts who did not have diabetes mellitus or other ocular diseases.
After the eye had been prepared in a standard fashion using 5% povidone/iodine and topical antibiotics, we obtained 0.1 to 0.2 ml of undiluted aqueous humor by limbal paracentesis using a 30-gauge needle attached to a microsyringe. We aspirated the aqueous humor within two to five seconds from the central pupillary area without touching the iris, lens, or corneal endothelium. The samples were placed immediately in liquid nitrogen and stored at -70℃ until analyzed. A total of 0.05 mL (1.25 mg) of undiluted bevacizumab was injected intracamerally through the limbus. After the injection, IOP and retinal artery perfusion were assessed, and patients were instructed to administer topical antibiotics (levofloxacin) for three days.
Aqueous sampling and bevacizumab injection were repeated after two weeks. Patients with established NVG were treated with medical and/or surgical therapy as needed. Ophthalmic evaluation included complete ophthalmic examination including degree of iris neovascularization and IOP, iris angiography, specular microscopy and slit lamp photography. The patients' records were reviewed to record patient demographics and clinical data.
Total VEGF concentrations were determined by ELISA (Cat. DVE00, R&D Systems, Minneapolis, MN USA) according to the manufacturer's instructions. The intensity of color developed was measured using an ELISA reader (MQX-200, Bio-TEK Instruments, Inc.) at 450 nm optical density (OD) with correction at 570 nm.
Before injection and two weeks after injection, corneal endothelial density, coefficient of variation and hexagonality were measured with specular microscopy, and intraocular pressure was measured with a Goldmann applanation tonometer.
The mean VEGF concentration in the aqueous humor in eyes with NVG was 1181.8±1248.3 pg/mL (46.0 to 2745.7 pg/mL) before intracameral injection of bevacizumab and that of the control group was 20.7±12.4 pg/mL. Two weeks after injection, the VEGF concentrations in the test group had decreased to 33.2±12.2 pg/mL(14.0 to 43.7 pg/mL) (p-=0.04) (Fig. 1). IOP was 29.2±10.1 mmHg before bevacizumab injection, 30.8±8.4 mmHg right after injection and dropped to 24.0±7.3 mmHg 2 weeks after injection, but the differences in these levels were not significant.
On specular microscopy examination, corneal endothelial density, coefficient of variation and hexagonality were 2246±552, 37.8±6.3, 52.8±12.1 respectively before bevacizumab injection and 2254±589, 38.2±8.1, 49.2±12.1 respectively two weeks after injection (p>0.05) (Table 1).
High-quality iris fluorescein angiographs were obtained before and after intracameral bevacizumab injection in five eyes. Two weeks after injection of bevacizumab, substantial regression of neovascularization or fluorescein leakage was seen in all eyes (Fig. 2, 3).
No complications developed after injection of bevacizumab, such as uveitis, endophthalmitis, effects of ocular toxic effects, or other obvious systemic adverse events. Blood pressure was monitored before initial injection and at each follow-up, and no significant elevations were observed over the course of the study.
Iris neovascularization with secondary angle-closure glaucoma is a serious sequela of a number of disease processes affecting the eye. Clinical studies indicate that diabetic retinopathy, retinal venous obstruction, and sickle cell disease are the leading etiologic factors for the development of NVG.1, 2, 9 It is now evident that several mediators are involved in the process of neovascularization, the most important and well studied of which is the VEGF-A.10-12
Ramesh et al. found that patients with NVG had significantly increased levels of VEGF in the aqueous humor, 40- and 113-fold higher than in patients with POAG or cataract, respectively, and implicated VEGF as an important factor in the pathogenesis of intraocular neovascularization.7
In animal studies, Tolentino et al. concluded that intravitreal injections of recombinant human VEGF165 in amounts comparable to those measured in eyes with active neovascularization are sufficient to produce noninflammatory iris neovascularization in nonhuman primates and that prolonged exposure to VEGF165 can produce neovascular glaucoma.13
Regarding the pivotal role of VEGF-A in ocular neovascularization, inhibition of this mediator appears to have a strong biologic basis for treatment of NVG.10-12
Shahin et al. reported two cases with neovascular glaucoma secondary to ischemic entral retinal vein occlusion who received treatment with intravitreal bevacizumab (Avastin), which is a nonselective antibody against VEGF-A. Both patients demonstrated dramatic short-term response in terms of intraocular pressure reduction and regression of neovascularization.10
In another case report, Milko et al injected bevacizumab intravitreally to six consecutive patients with NVG and intravitreal bevacizumab resulted in a marked regression of anterior segment neovascularization and relief of symptoms within 48 hours. IOP also decreased substantially in three eyes without further treatment.14
In a case of intravitreal bevacizumab injection in a patient with early stage neovascular glaucoma without peripheral anterior synechia, the visual acuity, IOP, regression of the iris and the angle of neovascularization were measured up to the 29 weeks after injection. Regression of the iris and angle of neovascularization were confirmed from the second week after injection.15
In a study of intracameral bevacizumab injection, 16 eyes of 15 patients with iris neovascularization associated with or without neovascular glaucoma secondary to proliferative retinal vasculopathies received intracameral bevacizumab (1.25 mg) and all patients had complete remission of the neovascularization within three weeks after the injection. Intraocular pressure was controlled with maximum medical therapy in eight of nine eyes reducing the need for glaucoma surgery.2
In our study, we injected bevacizumab in the anterior chamber of 5 NVG subjects. Two weeks after injection, in all the eyes, the leakage from iris neovascularization was decreased and engorged vesselswere regressed on an iris fluorescein angiogram. It was a similar result to that of other studies, but concerning intraocular pressure, there was no significant IOP lowering effect two weeks after injection.
Osamu et al. in their study of intravitreal bevacizumab injection on NVG patients, found that the VEGF concentration in the aqueous humor averaged 326±125 pg/mL before intravitreal injection of bevacizumab and decreased to less than 31 pg/mL in all eyes one week after injection, about a 10 fold decrease.16
In our study, VEGF levels were remarkably lowered to 33.2 pg/mL after intracameral bevacizumab injection, at least a 30 fold decrease, much higher than that seen after intravitreal injection. Reduced neovascularization may lead to a decrease in release of inflammatory cytokines from theiris and retinal vessels of neovascular glaucoma patients, and reduce the occurrence of peripheral anterior synechia.
Considering that our study measured VEGF levels two weeks after injection, the decrease in intracameral VEGF levels that we observed suggest that subsequent intracameral injections may be delayed for more than two weeks and still constitute effective treatment. Comparing these results to the effects of intravitreal injection, the amount of bevacizumab necessary could be reduced for intracameral injections.
In a prospective case series, Chiang et al. injected 2.5 mg of bevacizumab intravitreally into 50 eyes of 50 patients. Corneal endothelial cell densities of treated and untreated eyes before injection and at three and six months after injection were not significantly different. They concluded that intravitreal injection of 2.5 mg bevacizumab seemed to have no harmful effects on the corneal endothelium.17
Also, in cultured human corneal cells, there were no cytotoxic effects of bevacizumab on corneal keratinocytes, corneal fibroblasts, and corneal endothelial cells when used at concentrations of 5.0 mg/mL or lower. Bevacizumab-treated cells showed no signs of cellular damage compared with the control.18
In our study, two weeks after intracameral bevacizumab injection, there was no significant progression in polymegathism and pleomorphism of corneal endothelial cells, thus bevacizumab has no harmful effects on corneal endothelial cells. But a longer follow-up is needed to determine the safety of intracameral bevacizumab.
In conclusion, intracameral bevacizumab injection can reduce iris neovascularization in NVG patients. VEGF levels were significantly decreased two weeks after injection and corneal toxicity did not occur during the short term follow-up period.
REFERENCES
1. Tripathi RC, Li J, Tripathi BJ, et al. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology 1998;105:232-237.


2. Chalam KV, Guphta SK, Grover S, et al. Intracameral Avastin dramatically resolves iris neovascularization and reverses neovascular glaucoma. Eur J Ophthalmol 2008;18:255-262.


3. Glaser BM. Extracellular modulating factors and the control of intraocular neovascularization. Arch Ophthalmol 1988;106:603-607.


4. Sivalingam A, Kenney J, Brown GC, et al. Basic fibroblast growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol 1990;108:869-872.


5. Tripathi RC, Borisuth NSC, Li J, et al. Growth factors in the aqueous humor and their clinical significance. J Glaucoma 1994;3:248-258.


6. Meyer-Schwickerath R, Pfeiffer A, Blum WF, et al. Vitreous levels of the insulin-like growth factors I and II, and the insulin-like growth factor binding proteins 2 and 3, increase in neovascular eye diseases: studies in nondiabetic and diabetic subjects. J Clin Invest 1993;92:2620-2625.



7. Tripathi RC, Li J, Tripathi BJ, et al. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology 1998;105:232-237.


8. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin formetastatic colorectal cancer. N Engl J Med 2004;350:2335-2342.


9. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480-1487.


10. Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular Glaucoma. J Glaucoma 2007;16:437-439.


11. Sivack-Callcott JA, O'Day DM, Gass DM, et al. Evidence based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology 2001;108:1767-1776.


12. Ng EW, Anthony AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol 2005;40:352-368.


13. Tolentino MJ, Miller JW, Gragoudas ES, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol 1996;114:964-970.


14. Milko E, Domig Diego, Wolf-Schnurrbursch Ute, et al. Intravitreal Bevacizumab (Avastin®) in the Treatment of Neovascular Glaucoma. Am J Ophthalmol 2006;142:1054-1056.


15. Maeng Hyosung, Kim Jinchul, Kee Changwon, et al. Intraviteal Bevacizumab (Avastin® Injection for the Treatment of Early-Stage Neovascular Glaucoma. J Korean Ophthalmol Soc 2008;49:696-700.

16. Sawada O, Kawamura H, Kakinoki M, et al. Vascular Endothelial Growth Factor in Aqueous Humor Before and After Intravitreal Injection of Bevacizumab in Eyes With Diabetic Retinopathy. Arch Ophthalmol 2007;125:1363-1366.


Fig. 1
Vascular endothelial growth factor (VEGF) in the aqueous humor of eyes with neovascular glaucoma. VEGF levels are shown before intracameral bevacizumab injection (pre-bevacizumab), two weeks after injection (post-bevacizumab) and for the control group.
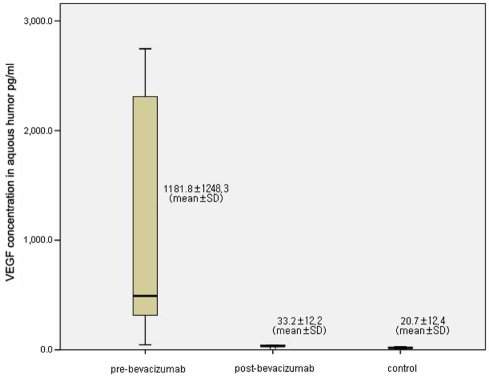
Fig. 2
(A) A NVG patient showing iris neovascularization before bevacizumab injection. (B) Two weeks after intracameral bevacizumab injection, neovascularization had completely regressed.

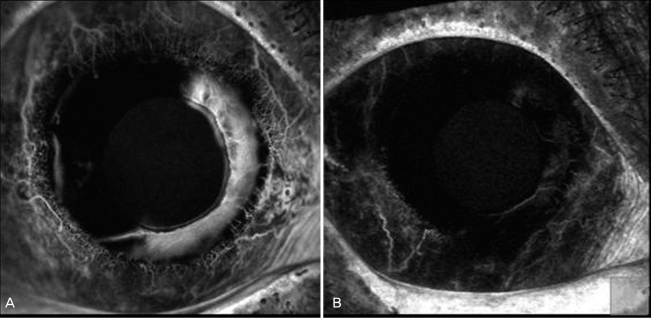
Fig. 3
(A) Iris fluorescein angiogram shows marked dye leakage from rubeotic vessels before intracameral vevacizumab injection. (B) Two weeks after intracameral bevacizumab injection, the leakage from iris neovascularization was remarkably decreased.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print