Clinical Evaluation of Accommodative Intraocular Lens Implantation in High Myopic Eyes
Article information
Abstract
Purpose
To compare the clinical outcome of AT-45 implantation between high myopic eyes and non-high myopic eyes.
Methods
Retrospective, non-randomized, comparative trial. The medical charts of 28 patients with 35 eyes who had phacoemulsification and AT-45 implantation were retrospectively reviewed. 13 eyes of 10 patients were included in the high myopic group (axial length ≥ 26.0 mm) and 22 eyes of 18 patients were included in the non-high myopic group. The clinical data included unilateral best-corrected visual acuity (BCVA) and distance-corrected near visual acuity (DCNVA) at 6-months follow-up after the surgery. The results were compared between the two groups.
Results
In the non-high myopic group, 22 eyes (100%) and 19 eyes (86.4%) achieved a BCVA of 20/25 and 20/20 or better respectively. For the high myopic group, the results were 13 eyes (100%) and 12 eyes (92.3%) respectively, at 6 months after the surgery. In the non-high myopic group, 21 (95.4%) and 7 eyes (31.8%) achieved a DCNVA of 20/40 and 20/25 or better. For the high myopic group, the results were 13 (100%) and 4 eyes (30.8%) respectively, at 6 months after the surgery, the differences between the two groups for a BCVA of 20/25 or better and 20/20 or better and a DCNVA 20/40 or better and 20/25 or better were not statistically significant.
Conclusions
Six months clinical outcome of cataract surgery with an AT-45 for the high myopic eyes was satisfactory; it was not significantly different from that of the non-high myopic eyes.
Over the past several decades, cataract surgical technique and intraocular lens (IOL) technology developed dramatically. Many of the surgical complications have been resolved. However, despite the excellent restoration of visual acuity and the biocompatibility of the IOLs, the postoperative near vision in the pseudophakic eye remains unsatisfactory. Therefore, most of the patients who have cataract extraction require glasses for near vision. To solve this problem, technologies for restoring near vision have been investigated and special IOLs have been developed.1-4
A recently developed accommodative IOL, the AT-45 (Eyeonics Vision, USA) has shown satisfactory clinical results and obtained U.S. Food and Drug Administration (FDA) approval in November 2003. However, in a FDA approved clinical trial, the longest axial length was only 26.6 mm and the clinical outcome after AT-45 implantation for high myopic eye has not been studied.
The purpose of this report was to evaluate the clinical outcome after AT-45 implantation for high myopic eyes and compare the results with non-high myopic eyes. Previously, we reported clinical outcome of the AT-45 in non-high myopic eyes.5 The same eyes of the previous study were included in the control group in this study.
Materials and Methods
The medical records of 29 patients with 36 treated eyes, who had phacoemulsification and AT-45 implantation with a minimum follow up of 6 months were retrospectively reviewed. None of the patients had a prior history of ocular trauma, eye surgery, intraocular inflammation, glaucoma, prominent retinal abnormalities or other ocular lesions that could affect the visual outcome. For the IOL power calculation, the immersion type A-scan biometer (Hiscan, Optikon, Italy), a manual keratometer (OM-4, Topcon, Japan) and the SRK/T calculation formula were used for the assessments. Eyes with an axial length of 26.0 mm or more were included in the 'high myopic group' and all others were included in the 'non-high myopic group'. The results of the non-high myopic group were used as a 'control' for evaluating the clinical outcome of AT-45 implantation in the high myopic eyes. Four eyes in the high myopic group, the target refraction was selected to be myopic, with the patients' agreement, because the lowest commercially available power of AT-45 was only +10.0 diopter (D).
One surgeon, ES Chung, performed all of the procedures, which were carried out using topical anesthesia. A 3.5 mm temporal clear corneal incision was performed and the capsulorhexis was sized at 5.0~5.5 mm. Careful phacoemulsification and polishing of the capsule were carried out to prevent posterior capsular rupture and opacity. During the operation, a proper volume of viscoelastic material was injected into the chamber to avoid hyperinflation of the posterior capsule. After the AT-45 was implanted into the capsular bag, the incision site was closed with 1 bite of a 10-0 nylon suture (Alcon, USA) to avoid postoperative aqueous leakage. Two hours after the surgery, slit lamp examination was performed to assess the IOP, postoperative aqueous leakage and the IOL position.
Postoperative medications included 0.5% levofloxacin (Cravit, Santen, Japan), prednisolone acetate (1% Predforte, Allergan, USA) and cyclopentolate HCL (1% Cyclogyl, Alcon, USA). One drop of atropine (1% Ocutropine, Samil, Korea) was applied just after the surgery and cyclopentolate was used for 10 days after the surgery. Two weeks after the procedure, all the patients received near vision training. Postoperative evaluations including the slit lamp examination, manifest refraction (at 2 weeks, 1, 2 and 6 months following surgery), best-corrected visual acuity (BCVA) and distance-corrected near visual acuity (DCNVA), which is near visual acuity with distance correction (at 6 months after surgery) were performed. Distance and near visual acuities were measured with the ETDRS visual acuity chart and the Jaeger chart respectively. All the study outcomes were compared between the high myopic and non-high myopic groups.
Statistical analysis was performed using a commercially available statistical package (SPSS ver. 11.5 for Windows; SPSS Sciences, Chicago, USA). Differences in the BCVA and the DCNVA between the two groups were evaluated with the Fisher's exact test. The independent samples T-test was used to evaluate changes in the SE between the two groups. For all statistical analyses, a P<0.05 was considered significant.
Results
Twenty three eyes of 19 patients were classified as non-high myopic (axial length < 26.0 mm) and 13 eyes of 10 patients were considered high myopic (axial length ≥ 26.0 mm). All the operations were successful without any intraoperative complications. Complications such as immediate postoperative aqueous leaking or IOL decenteration were not observed. One eye of the non-high myopic group showed +1.5D of hyperopic shift at 2 weeks following surgery. The patient complained about visual disturbance and piggybacking using monofocal IOL was performed at 4 weeks following the initial surgery. This case was included in the analysis of complication and predictability, but excluded from the visual acuity and spherical equivalent analysis. Therefore, 22 eyes of 19 patients were included in the non-high myopic group for visual acuity and spherical equivalent analysis.
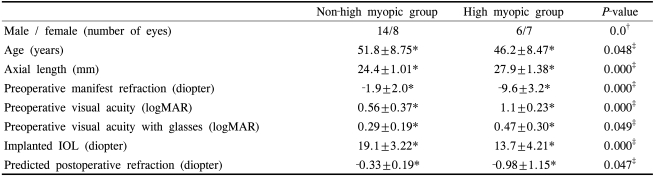
The mean age of the patients was 51.8±8.8 years (range 38 to 72 years) in the non-high myopic group and 46.2±8.5 years (range 29 to 61 years) in the high myopic group (Independent samples T-test, P=0.048). The mean axial length was 24.4±1.0 mm and 27.9±1.4 mm (Independent samples T-test, P=0.000), the mean implanted IOL power was 19.1±3.2 D and 13.7±4.2D (Independent samples T-test, P=0.000) and the mean predicted postoperative refraction was -0.33±0.19D and -0.98 ±1.15D respectively (Independent samples T-test, P=0.047) (Table 1).
Six months after the surgery, a BCVA of 20/25 or better was achieved in 22 (100%) of the non-high myopic group and in 13 (100%) of the high myopic group.
A BCVA of 20/20 or better was achieved in 19 (86.4%) of the non-high myopic group, and in 12 (92.3%) of the high myopic group (Fig. 1). A DCNVA of 20/40 or better was achieved in 21 (95.4%) of the non-high myopic group, and in 13 (100%) of the high myopic group. A DCNVA of 20/25 or better was achieved in 7 (31.8%) of the non-high myopic group and in 4 (30.8%) of the high myopic group (Fig. 2). Differences among the non-high myopic and high myopic groups in achieving the BCVA of 20/25 or better (Fisher's exact test, P=1.000) and 20/20 or better (Fisher's exact test, P=1.000) were not statistically significant. In addition, the differences in the DCNVA of 20/40 or better (Fisher's exact test, P=1.000) and a 20/25 or better (Fisher's exact test, P=1.000) were not statistically significant.

Best-corrected distance visual acuity in the non-high myopic group (n=22) and the high myopic group (n=13), 6 months after AT-45 implantation.
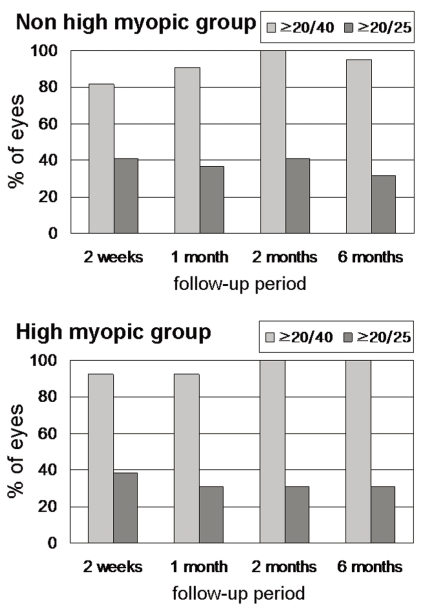
Distance-corrected near visual acuity in the non-high myopic group (n=22) and in the high myopic group (n=13), 6 months after AT-45 implantation.
The mean spherical equivalent (SE) change during the follow up period was -0.25±0.48D (2 weeks), -0.22±0.29D (1 month), -0.14±0.39D (2 months) and -0.16±0.36D (6 months) in the non-high myopic group and -0.92±1.06D (2 weeks), -0.57±0.97D (1 month), -0.47±1.03D (2 months), -0.64±0.89D (6 months) in the high myopic group. The mean SE difference between postop 2 weeks and 6 months postoperatively was 0.09±0.39D in the non-high myopic group and 0.28±0.67D in the high myopic group (Table 2). The SE difference, in comparison between the two groups, was not statistically significant (Independent samples T-test, P=0.429).
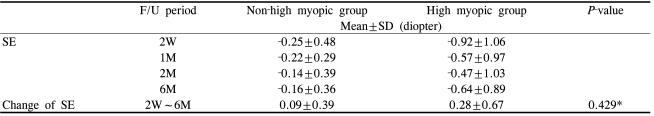
Mean spherical equivalent (SE) change after AT-45 implantation in the non-high myopic group (n=22) and the high myopic group (n=13), during follow-up (F/U). The difference in SE between postoperative 2 weeks and 6 months was not statistically significant in comparison between the two groups (P=0.429)
For the non-high myopic and high myopic groups, 20 eyes (90.9%) and 12 eyes (92.3%) were within 1.0D of the predicted postoperative SE (Fisher's exact test, P=1.000) and 17 eyes (72.7%) and 8 eyes (61.5%) were within 0.5D of the predicted postoperative SE respectively (Fisher's exact test, P=0.888).
There was no acute postoperative abnormality by slit lamp biometry observed in any of the cases. In the non-high myopic group, postoperative complications were noted in 7 eyes (31.8%). As mentioned above, one case of significant postoperative hyperopic shift accompanied with posterior capsular fold was noted in 1 eye. Five cases had posterior capsular opacities develop and 3 cases required Nd:YAG laser posterior capsulotomy. One case had posterior synechia develop, which treated successfully by synechiolysis using a clear corneal incision. In the high myopic group, postoperative complications were noted in 3 eyes (23.1%). Two cases had posterior capsular opacities develop but they did not require further treatment. One case had asymmetric vaulting with posterior capsular fibrosis and contraction developed at 1 month post surgery. After Nd:YAG laser posterior capsulotomy, the position of the IOL was normalized.
Discussion
Theoretically, the accommodation after cataract surgery with monofocal IOL implantation is not sufficient to achieve satisfactory near vision. However, despite the pseudophakic state, there are some cases with good postoperative near vision. This phenomenon is not a 'true-accommodation' but rather a 'pseudo-accommodation', that is associated with the pupil size, residual myopia and astigmatism.6,7
Cumming and Kammann postulated that the increased anterior vitreous pressure, which is triggered by contraction and mass redistribution of the ciliary body, could change the refractive power of the eye. This can occur by inducing anterior movement of the IOL, and AT-45 was developed based on this hypothesis.8-12 Several studies have been reported that support this hypothesis by demonstrating intraocular movement of IOL.13,14 However, the true mechanism of action of the AT-45 has not been confirmed.
Although still debated, the overall reported clinical outcome after the AT-45 implantation has been relatively satisfactory.12,15-20 When compared with other available IOLs for near vision, the AT-45 has shown similar or better near vision performance.20-24 However, the visual outcome after cataract surgery may be influenced by various factors related to the patient and surgeon and the methods and location of patient evaluation at follow-up. Furthermore, for the recently developed IOL, the study population to date may still be small and the follow-up period short. Therefore, such limitations must be considered when interpreting the reported study results.
Previously, most of the patients who received an AT-45 implantation had a normal axial length. According to the manufacturer's instructions (Crystalens vision enhancement course material, Eyeonics, Inc., 2004), there is a weak correlation between the axial length and the accommodative effect and the clinical outcome in myopic eyes was said to be as good as that of the non-myopic eyes. However in the FDA clinical trial, the highest axial length was only 26.6 mm. In addition, the function of the AT-45 was not sufficiently evaluated in high myopic eyes.19 After FDA approval, even until recently, the clinical outcome of AT-45 in high-myopic eyes has not been reported. In the United States and Europe, only a small portion of the population has high myopia. However, the incidence of high myopia in Asia is much higher than in other ethnic groups.25-28 Therefore, the evaluation of the AT-45 in high myopic eyes is especially important for Asian populations. The conventional definition of high myopia is a SE of less than -6.0D. In our study, to exclude lenticular myopia, we defined high myopia as an axial length of 26.0 mm or more, according to the Pericival's definition.29
In this study, the postoperative distance and near visual acuity as well as predictability of the postoperative refraction of the high myopic eyes were satisfactory and were not significantly different from those of the non-high myopic eyes. Compared to emmetropic eyes, high myopic eyes have several distinct characteristics, which may have adverse effects on the clinical outcome after AT-45 implantation. High myopic eyes typically have a deeper anterior chamber and the vitreous body is frequently more liquefied than in a similar-aged emmetrope. Therefore, excessive anterior chamber deepening and hyperinflation of the posterior capsule may occur during surgery. According to the manufacturer's instructions (Crystalens vision enhancement course material, Eyeonics, Inc., 2004), hyperinflation of the posterior capsule may change the post-operative refraction leading to a more hyperopic result by influencing the position of the AT-45. Moreover, the IOL power determination in high myopic eyes is relatively inaccurate and this may lead to postoperative hyperopia resulting in a significant effect on the near vision. In order to reduce this possible error, we chose the immersion type A-scan biometry for axial length measurement, which has several advantages over contact type A-scan biometry.30,31 All A-scan biometry was performed by one experienced technician. We applied the SRK/T formula for both non-high myopic and high-myopic groups based on the recommended calculation formula for AT-45 implantation with SRK-T (Crystalens vision enhancement course material, Eyeonics, Inc., 2004). This formula is also recommended for eyes with axial myopia.32,33
Prior reports have demonstrated that the accommodative range induced by forward IOL movement varies depending on the axial length and power of the implanted IOL. Short eyes with high IOL power have shown a more effective accommodation with an IOL shift.34 Therefore, the results of this study suggest that pseudoaccommodation may partially affect near vision after AT-45 implantation.
The findings of this study showed that both groups had continuous hyperopic changes for the first 2 months after the surgery. Although the changes were slightly more prominent in the high myopic group, the differences between the two groups were not statistically significant. Because the target refractions were myopic in 4 eyes, in the high myopic group, the mean SE of the high myopic group had a more negative value than that of the non-high myopic group. However, the difference in the SE at 2 weeks and 6 months after surgery was not statistically significant in comparison between the two groups.
The overall complication rates in the two groups were similar. A relatively high incidence of a posterior capsular opacity was noted in both groups. However, less than half of the cases complained of decreased visual acuity. In the high myopic group, one case of asymmetric vaulting with posterior capsular contraction developed, which is a specific complication of AT-45 implantation.35,36 Prior reports have shown asymmetric vaulting at 3 weeks35 and 6 months36 after surgery. Asymmetric bag fibrosis35 and central posterior capsular opacity with a fibrotic band36 was suspected as the cause of the IOL vaulting. Similarly, posterior capsular fibrosis and contraction was noted in our cases, this was successfully treated with Nd:YAG laser posterior capsulotomy. One eye in the non-high myopic group showed significant hyperopic shift two weeks postoperatively. This may be attributed to the contraction of posterior capsule, although the reason of this finding was not clearly revealed yet, as mentioned in a previous report.5
It has been several years since FDA approved AT-45 implantation. However, the performance and complications associated with AT-45 implantation are not yet fully understood. The results of our study showed that AT-45 implantation for high myopic eyes was safe and effective and the outcomes were not significantly different from non-high myopic eyes. Further studies with a larger patient population and a prospective and randomized study design as well as a longer follow up are required for confirmation of the clinical outcome after AT-45 implantation in high myopic eyes.
Notes
XXIV Congress of the ESCRS, London, England, 9-13 September 2006.