Short-term Efficacy of Topical Immunosuppressive Agents on the Survival of Cultivated Allo-Conjunctival Equivalents
Article information
Abstract
Purpose
To investigate the short-term efficacy of topical immunosuppressive agents on the survival of cultivated allo-conjunctival equivalents.
Methods
Twenty-five eyes of New Zealand white rabbits were included. Temporal conjunctivae were trephined to a diameter of 7.5 mm, and then cultured allo-conjunctival epithelial cells on amniotic membrane were transplanted onto them. Various immunosuppressants including steroid, cyclosporine, and rapamycin were applied topically four times a day for a week. Epithelial defects and graft edema were graded daily. Numbers of inflammatory cells were measured in H&E. PKH26 and cytokeratin 4 and 7 were immunostained.
Results
Earlier epithelialization was observed in 1% steroid-treated eyes and defects persisted significantly in 0.5% CsA applied eyes. In histology, PKH26 positive cells considered as donor cells were only found in 1% steroid or 0.01% rapamycin applied eyes. 1% steroid- or 0.01% rapamycin-applied eyes both showed positive staining for keratin-4 and -7. Inflammatory cells were less found in 1% steroid or 0.01% rapamycin treated eyes.
Conclusions
Topical steroid or rapamycin can help to suppress acute inflammation and enhance the acute survival of transplanted conjunctival cells.
Conjunctival transplantation in conjunctiva depleting diseases has been described for several decades.1-4 However, most conjunctival allografts have failed because they are rejected more easily than corneal allografts. The immunosuppressive agents have been used in order to reduce the rejections. Although systemic immunosuppressive agents have multiple systemic side effects,5 systemic immunosuppression is known to be needed for the survival and maintenance of allo-conjunctival grafts.6 The mechanisms of immunosuppressive agents are different from each other. Steroid and rapamycin inhibit various types of inflammatory cells and neovascularization.7-9 Cyclosporin has been known that it inhibits T and/or B cell and can retard the corneal neovascularization but is ineffective in blocking vessel growth in the subcutaneous disc angiogenesis system.10
Recently, a study reported that the cultivation of cells reduced antigenicity,11 and the transplantation of cultivated allo-conjunctival cells has been attempted. We thought that these reduced antigenicity may allow the systemic immunosuppressive agents to be unnecessary or little necessary with topical application of immunosuppressive agents.
The penetrations of topical immunosuppressants including cyclosporine, FK506, and rapamycin have been investigated in animal models, which showed variable results across the studies.12-20
We hypothesized that topical immunosuppressants may affect the survivals of conjunctival and corneal grafts differently, even if deep penetration by drugs may not be achieved. Therefore, we investigated the efficacies of various topical immunosuppressive agents on the survival of transplanted cultivated allo-conjunctival cells (conjunctival equivalents) in this study.
Materials and Methods
Animals
All procedures were performed according to the ARVO Statement for the use of Animals in Ophthalmic and Vision Research. New Zealand white rabbits of both sexes aged between 4 and 6 months were used in all experiments. Rabbits were sacrificed by an intramuscular injection of 50 mg xylazine hydrochloride and 50 mg ketamine hydrochloride followed by an intravenous overdose of sodium pentobarbital. Twenty-five eyes were allocated to five groups according to treatment modality.
Conjunctival equivalents construction
We cultured rabbit conjunctival epithelial cells using a previously described culture system for conjunctival epithelial stem cells, with several modifications.21,22 Briefly, rabbit conjunctival tissues were treated with 0.05% trypsin and 0.01% ethylenediaminetetraacetic acid (EDTA) at 37℃ with gentle shaking (HB-Movcst 220, Hybaid Limited, U.K). Suspended cells were collected every 20 minutes 4 times. After plating NIH3T3 feeder cells which were treated with 4 µg/ml mitomycin C (MMC) at 37℃ for 2 hr, epithelial cells (1.5-1.8×104 cell/cm2) were seeded onto human denuded amniotic membrane (AM) in culture inserts and co-cultured in 5% CO2 at 37℃. The medium used consisted of SHEM supplemented with 10% FBS (Gibco, USA) in DMEM/F12 (1:1) (Gibco, USA), 0.5% PS (Gibco, USA), 4 mM L-glutamine (Sigma, USA), 5 ng/ml EGF (Sigma, USA), 5 µg/ml insulin (Sigma, USA), 30 ng/ml choleratoxin (Sigma, USA), 0.18 mM adenine (Sigma, USA), 2 nM 3.3', 5 triiodo-L-thyromine sodium salt, and 0.4 µg/ml hydrocortisone. Epithelial cells were submerged in the medium and cultured for 2 weeks. All epithelial sheets on AMs were well maintained and showed no phenotypical differences.
Surgical Procedure
Cultivated cells on denuded AMs for 2 weeks were used as cultivated conjunctival equivalents. Temporal conjunctivae were trephined to a diameter of 7.5 mm. Cultivated conjunctival equivalents were transplanted to the defected conjunctival lesion in recipients (Fig. 1) and sutured with interrupted and continuous 10.0 nylon. AMs were overlaid using 10.0 nylon to help graft stability.
Treatment
Each eye was treated four times daily with topical application of 1% steroid (Pred Forte®, Allergen, USA, n=5), 0.5% cyclosporine A (n=5), or 0.01% rapamycin (n=5) or with no topical treatment (controls; n=5). These represented the five topical treatment groups. 0.5% cyclosporine A eye drops was prepared with emulsifying cyclosporine A (Sandimun, Novatis, USA) in normal saline. Topical rapamycin (Sigma, USA) was mixed with phosphate buffer solution (PBS) and diluted to 0.01%.
Clinical Graft Evaluation
Each rabbit was examined daily under a microscope for 7 days after conjunctival equivalent transplantation. A single masked observer scored each conjunctival equivalent graft under the microscope using scales of epithelial defect and graft edema. Epithelial defect was measured by fluorescein staining % of entire grafts, and graft edema was scored as 0 (clear graft), 1 (mild opacity, with clearly visible episcleral vessels), 2 (moderate edema partly obscuring episcleral vessels), 3 (severe opacity totally masking episcleral vessels).
Histology
Animals were sacrificed 7 days after transplantation. Cryosections (5 µm) of the transplanted grafts per animal were cut and stained with H&E. To evaluate the presence of transplanted epithelial and goblet cells, indirect immunofluorescence microscopy was performed using monoclonal anti-keratin 4 (Vector, USA) as a marker for conjunctival epithelial cell or anti-keratin 7 (Vector) as a marker for goblet cell. Briefly, cryostat sections (5 µm) were placed on gelatin-coated slides and acetone-fixed at room temperature for 15 minutes. Sections were then treated with proteinase K 20 µg/ml for 10 minutes and washed twice with PBS. To block endogenous peroxidase activity sections were incubated in 0.5% H2O2 for 10 minutes and then washed with PBS followed by PBS containing 0.3% triton X-100 (PBST; 3×5 minutes) and then incubated in PBS containing 1% serum for 30 minutes. Subsequently, sections were incubated at room temperature for 1 hour with primary antibody and then washed with PBS (2×5 minutes). The sections were then incubated at room temperature for 1 hour with Alexa 488 (A11001, Molecular Probes, USA) at 2 mg/ml. After staining with primary antibody, sections were incubated at room temperature for 1 hour with appropriate secondary antibodies and fluorescein (FIITC)-conjugated goat anti-mouse IgG (Molecular Probes). After several washing with PBS, sections were coverslipped using antifading mounting. Anti-keratin 4 or 7 antibodies were stained on amniotic membrane without cells as a negative control. For cell tracing, cultured conjunctival epithelial cells and allo-conjunctival cells were stained with 10 nM of a fluorescent dye (PKH26; Sigma, USA) in BSS (Alcon, USA) for 30 minutes at 37℃ and then washed with BSS (Alcon, USA) for 30 minutes at 37℃ one day before transplantation. Cryosections of regenerated conjunctiva were co-stained with a DNA-binding dye Hoechst (Sigma). The expressions of PKH26, keratin-4, and -7 were evaluated. PKH26 positive cells were regarded as donor cells.
Statistics
Differences between control and treatment groups were examined using the Mann-Whitney test. For all analyses, statistical significance was set at the α= 0.05 level. Data analysis was performed using SPSS for Windows® ver. 12.0 (SPSS, Chicago, USA).
Results
Clinical results
When compared between the control and the treatment group, the control group without immunosuppression showed decreasing conjunctival epithelial defects with time, while grafts in the treatment group were not epithelialized completely during the 7 day follow-up period (Mann-Whitney test, p=0.010 at day 7) (Fig 2A). Graft edema in the control group was significantly more severe on days 2 and 3 than in the treatment groups, while this was not significant at day 4, 5, 6, and 7 (Mann-Whitney test, p=0.071 at day 1, p=0.029 at day 2, p=0.019 at day 3, p=0.097 at day 4, p=0.371 at day 5, p=0.488 at day 6, and p=0.336 at day 7) (Fig 2B).
When compared among the treatment groups, epithelial defects tended to be decreased by steroids (Mann-Whitney test, p=0.056 in steroid treated eyes, and p=0.222 for rapamycin treated eyes at day 7) but defects persisted significantly more in 0.5% CsA applied eyes than in control eyes (Mann-Whitney test, p=0.008).
Histology
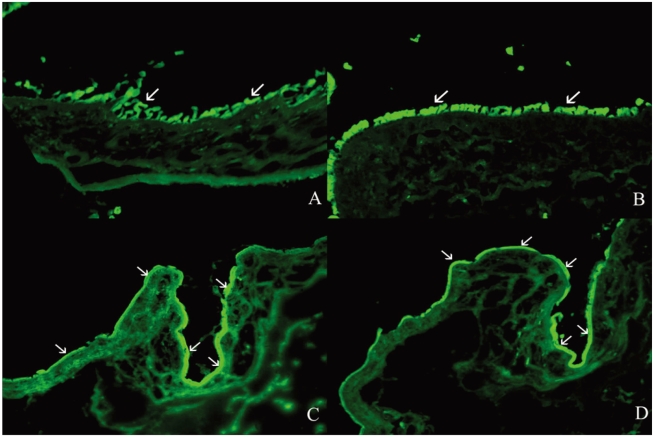
Histologic examination with H&E staining showed inflammatory cell infiltration was less severe in the 1% steroid or 0.01% rapamycin treated groups than in the control group (Mann-Whitney, p=0.000, and p=0.000 respectively) (Fig. 3, Table 1). Numbers of inflammatory cells were marked in 0.5% cyclosporine applied eyes.
Number of inflammatory cells in 1% steroid, 0.5% cyclosporine, or 0.01% rapamycin applied eyes in the cultivated conjunctival equivalent transplanted group. Histogram data represent means±SD. *p<0.05.
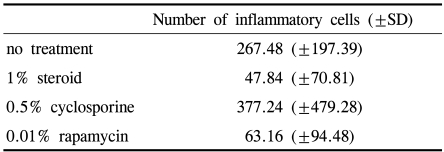
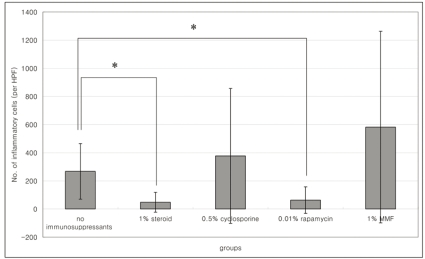
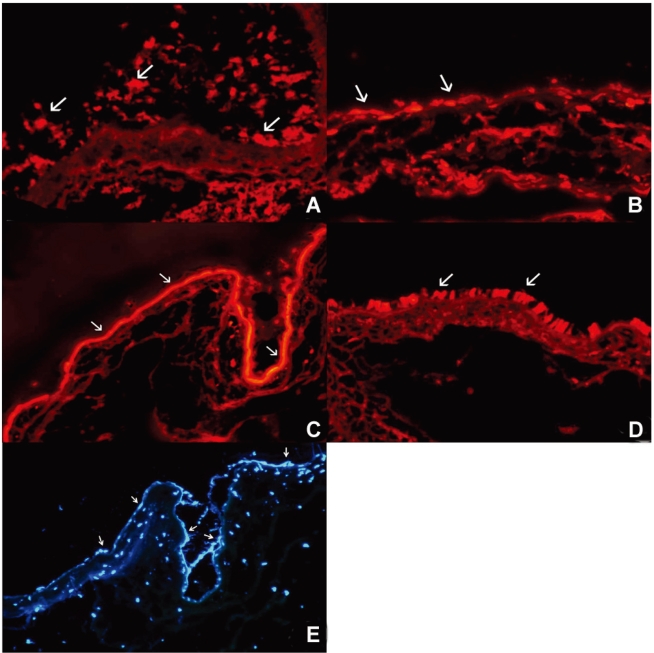
PKH26 staining showed no donor cells in the control without any immune suppressant or in 0.5% CsA treated eyes (Fig. 4A, B). PKH26 positive cells were found in 1% steroid, 0.01% rapamycin applied eyes (Fig. 4C, D), and 1% steroid or 0.01% rapamycin-applied eyes both showed positive staining for keratin-4 and -7 (Fig. 5A~D). We confirmed the cells using a DNA-binding dye Hoechst staining (Fig. 4E, F). Taken together, treatment with topical steroid or rapamycin enhanced grafted epithelial cell survival.
PKH26 positive cells as transplanted donor cells in untreated (A), and in 0.5% cyclosporine (B), 1% steroid (C), 0.01% rapamycin (D) treated eyes in the cultivated conjunctival equivalent transplanted group. Hoechst staining in steroid (E) or 0.01% rapamycin (F) treated eyes in the cultivated conjunctival equivalent transplanted group. Magnification; ×200.
Discussion
Perturbations of the conjunctival microenvironment may induce corneal damage, but it is difficult to restore the normal conjunctival environment after severe conjunctival damage resulting from inflammation or chemical insult. Allo-conjunctival transplantation in ocular surface disorders has been previously described as a trial in order to overcome these conditions.2 The major problem of conjunctival allografts is the remarkable level of rejection.2 Several immunosuppressive agents including cyclosporine A, steroids, and rapamycin were used systemically or topically in order to reduce the rejection.12-20 These immunosuppresseive agents have several side effects when they are used systemically.12-20
In the present study, we used human AM which has been described as a substitute of basement membrane.23 It has also been described that epithelial cells can be adhered to AM and can differentiate on the AM.23-26 Moreover, human AM has been also described to display very low immunogenecity, suggesting that acute rejection would not occur after transplantation.27,28 In addition, we cultured epithelial cells without the use of the murine 3T3 fibroblast in this study, suggesting that the donor grafts in this study were expected to present only alloantigens.
We evaluated the survival of donor cells after Allo-conjunctival equivalent transplantation using PKH26 tracer and investigated the effect of topical immunosuppressants on the graft survival. PKH26 is a membrane dye that can facilitate tracking of the donor cells, because it is a fluorescent compound that is incorporated into the cell membrane. Therefore, PKH26 is useful for in vitro cell labeling, and in vitro and in vivo cell tracking applications.29-33 The cells stained with PKH26 can be considered as donor cells or daughter cells from donor cells because we had stained donor cells before the transplantation.
The present study revealed earlier epithelialization and lower graft edema in 1% steroid-treated eyes after the transplantation of cultivated allo-conjunctival equivalents. Histology also showed less inflammatory cell infiltration, a healthy PKH26 positive cells population, and positive staining for cytokeratin 4 and 7 in 1% steroid or 0.01% rapamycin applied eyes after transplantation, suggesting that both might prolong the survival of grafted cells. Steroid suppresses an immune reaction and extends the survival of transplanted tissues by;34-36 stabilizing lysosomal membranes, suppressing prostaglandin synthesis, modulating the gene transcriptions of pro-inflammatory cytokines (IL-1, IL-2, IL-6, IFN-γ and TNF-α, via corticosteroid/glucocorticoid receptor complex), impairing monocyte/macrophage function, and decreasing the number of circulating CD4+ T cells. The application of topical rapamycin has been known to have a positive effect on immune suppression in various types of experimental models, e.g., on corneal allograft rejection and neovascularization in rats, and on endotoxin-uveitis in rabbits.34,37-39 Rapamycin binds to FK binding protein 25 and inhibits mTOR (mammalian target of rapamycin), as a result, it blocks the cell signaling required for cell-cycle progression and for the cellular proliferation of T, B, mast, and peripheral blood mononuclear cells, fibroblasts and others.40,41
CsA applied eye showed more inflammatory cell infiltration and little PKH 26 cells. The reason why CsA had no effect on the survival of grafted conjunctival cells could be that CsA is a specific T cell suppressant. CsA binds to cyclophilins, and the CsA cyclophilin complex blocks the calmodulin/calcineurin-induced phosphorylation of transcription factors (i.e., interleukin-2) that are involved in early T-cell gene expression.34 We believe that abundant vessels in conjunctivae can easily recruit neutrophils and other mononuclear cells during the early stage, thus the nonspecific inhibition of early inflammatory cells seems to be required, as well as the inhibition of T and B cells. Therefore, T and/or B cell inhibition by CsA is probably not enough in conjunctival grafts. On the other hand, steroid or rapamycin have the capacity to inhibit various types of inflammatory cells, exerting a potent effect on conjunctival grafts. Meanwhile, cyclosporine is known to have epithelial toxicity,42 and that is why transplanted epithelial sheet showed persistent epithelial defect with application of cyclosporin.
Generally, cultivated allo-conjunctival equivalents in this study showed less inflammatory cell infiltration. This agrees with a previously study which found that cultivated cells had reduced immunogenicity.11 However, recipient site should be well-vascularized for graft survival, and the conjunctiva has good lymphatic drainages from subconjunctival lymphoid tissues.43 As a result, immune rejection cannot be avoided even in cultivated cells in the absence of immunosuppressants. Therefore, it is remarkable that the topical application of 1% steroid or 0.01% rapamycin showed good immunosuppression and a positive effect on survival in cultivated allo-conjunctival equivalents, in view of the fact that the conjunctiva presents a high risk area of rejection.
We investigated the efficacy only for 7 days. It may not be enough time to investigate the effect on the full course of graft rejection. However, conjunctiva is fully vascularized tissue, thus early severe inflammatory responses are expected as well as late cell-mediated rejection. Hence, we believe the evaluation of short term effect of those agents on the early survival of transplanted conjunctival cells would be worthy of notice. Further study is pending to investigate the long-term efficacy of topical immunosuppressants.
In conclusion, topical steroid or rapamycin were found to help suppress early stage inflammation, and thus, to extend allo-conjunctival equivalent transplant survival, which suggests that these agents can be used instead of systemic immunosuppressants in patients that have undergone allo-conjunctival transplantation.
Notes
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea 02-PJ10-PG8-EC01-0032.