Owing to the small incision and lack of suture, 25-gauge TSV enables minimization of surgical trauma, removal of suture related inflammation, and faster postoperative recovery. However, many vitreoretinal surgeons use it only in limited indications due to the inborn high flexibility of 25-gauge instruments. TSV with 23-gauge, introduced by Eckardt in 2005,
3 offers a firmer instrument and supports easier use by the vitreous surgeon who is more familiar with the 20-gauge intrument rather than the 25-gauge instrument. This advantage may help the surgeon converting from 20-gauge vitrectomy to TSV. Even though its use is increasing, the efficacy and complications of 23-gauge TSV has not yet been determined due to the very recent introduction. To elucidate these aspects, we report our initial experience of 40 eyes of 40 patients who were treated with 23-gauge TSV to evaluate the surgical outcome and its complications.
Materials and Methods
Patients
We retrospectively reviewed the medical records of the 40 eyes of 40 patients who underwent 23-gauge TSV by single surgeon from June 2005 till March 2006. All patients gave their informed consent prior to their inclusion in the study and Institutional Ethics Committee was granted for the study. The indications for vitrectomy were idiopathic epiretinal membrane (7 eyes), vitreous hemorrhage (11 eyes), diabetic macular edema (10 eyes), macular hole (5 eyes), vitreomacular traction syndrome (5 eyes), diabetic tractional retinal detachment (1 eye), and rhegmatogenous retinal detachment (1 eye) (
Table 1). Complicated cases such as severe proliferative vitreoretinopathy (PVR), severe diabetic tractional detachment, expected silicone oil injection, trauma and intraocular foreign body were not included in this study.
Surgical method
Surgery with 23-gauge TSV was performed by the same method and instruments suggested by Eckardt. Briefly, the conjunctiva was displaced about 2 mm laterally using a specially designed pressure plate (DORC, Zuidland, Holland) and hold the eye firmly. A 15 to 30┬░ angled tunnel incision which was parallel to the limbus, and was made through the conjunctiva, sclera, and pars plana 3.5 mm from the corneoscleral limbus a with 23-gauge stiletto blade (45┬░ angle; BD Medical-Ophthalmic Systems, Franklin Lakes, NJ). The microcannula was then inserted through the tunnel incision already made using a specially designed, blunt inserter (DORC, Zuidland, Holland). Twenty three-gauge instruments such as vitrectomy probe, microscissors, forceps, endolaser probe, endodiathermy probe, and flute needle were used for vitrectomy. A pneumatic vitreous cutter was used with a vitrectomy unit (Accurus, Alcon Surgical, Texas, USA). Fluid gas exchange was performed in 9 eyes. Combined cataract extraction through clear corneal incision performed in 23 eyes. Patients with idiopathic macular hole underwent internal limiting membrane peeling. At the end of the surgery, the microcannulas were withdrawn using specially designed, cannular forceps (DORC, Zuidland, Holland) from scleral tunnel incision without suturing of the sclera and conjunctiva. Gentle pressing with cotton tip applicator was applied on the sclerotomy site to enhance sealing of sclerotomy and return the displaced conjunctive to its original position. If there was subconjunctival hemorrhage at the incision site, gentle pressure using a cotton tip applicator over the bleeding site was applied till the homeostasis occurred. Only 2 cases required diathermy through the previous conjunctival opening. Suture was applied at the sclerotomy site if any sign of leakage was found such as bleb formation or hypotony. Antibiotics and steroids were then injected into the remaining 1 quadrant of the subconjunctival space that was not served for sclerotomy.
Examinations and follow-up
Each patient underwent a thorough preoperative examination including Snellen visual acuity and applanation tonometry. Other ancillary tests such as fundus photography, optical coherence tomography, sonography, and fluorescein angiography were also performed if possible. Snellen visual acuity was converted into a logarithm of the minimum angle of resolution (LogMAR) score for analysis. The Wilcoxon signed rank test was used to compare the scores at the different postoperative periods. A p value of less than .05 was considered to indicate statistical significance.
Patients were evaluated postoperatively using the same preoperative tests. Goldman applanation tonometry was performed on the operation day at 2 and 5 hours after surgery, and again at one day, 1 week, 1 month, and 3 months postoperatively, and also at all follow-up visits after that period. Visual acuity was measured at 1 week, and 1, 3, and 6 months postoperatively, and also at all follow-up visits after that period. In cases of gas tamponade, visual acuity measurement was performed after disappearance of the intraocular gas.
Results
The average patient age at the time of surgery was 61 years (range, 41-74 years). Twelve patients were men. Twenty-two right eyes and 18 left eyes underwent surgery. The mean postoperative follow-up time was 8.4┬▒3.4 months (range 3-13 months)
No complications were encountered with cannula use in any of the 40 eyes, nor was any slippage or loss of the cannula evident during scleral indentation for visualization of the retinal periphery.
Combined phacoemulsification and posterior chamber intraocular lens (IOL) implantation through clear corneal incision was performed in 23 eyes. All the cataract procedures were performed before the sclerotomy incisions were placed. A transient 10-0 nylon suture at clear cornea incision helped to prevent anterior chamber collapse during the cannula insertion procedure. There was no failure in cannula insertion due to hypotony or eye ball collapse. We didn't observe any IOL-related complications during the operation and postoperative follow-up periods.
Intraoperative Suture placement was necessary in 3 eyes (7.5%). In one case of vitreous hemorrhage, sclerotomy of the dominant hand side was converted into a 20-gauge procedure to allow the use of 20-gauge endolaser probe due to impairment of the 23-gauge endolaser system. In one eye with Terson syndrome, leakage occurred at all 3 sclerotomy sites and in the other eye comprising vitreous hemorrhage with branched retinal vein occlusion, leakage occurred at the dominant hand site after vitrectomy.
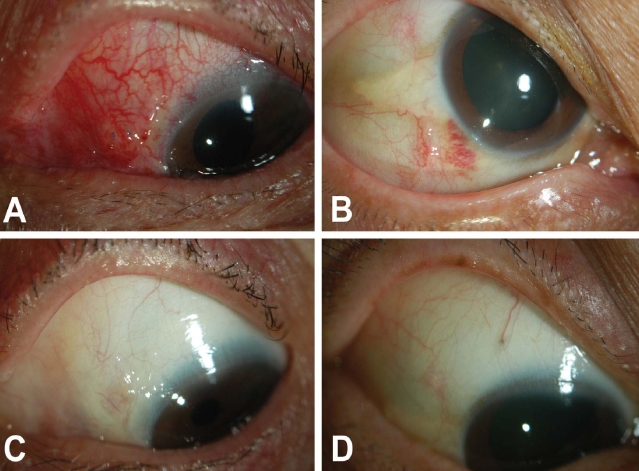
Postoperative discomfort was reduced to a mild level as early as the first postoperative day in most cases and a much diminished traumatic external appearance was observed with the 23-gauge system compared with external photographs after vitrectomy with the 20-gauge system (
Fig. 1).
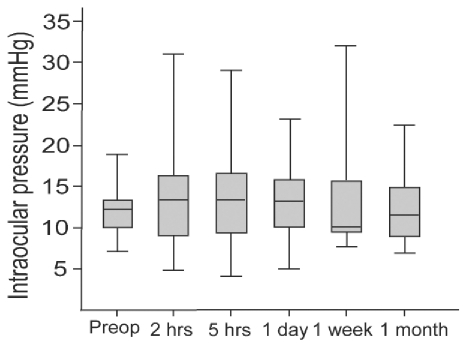
The mean preoperative intraocular pressure (IOP) of all 40 eyes was 12.0┬▒2.7 mmHg (range: 7-19 mmHg) and postoperative IOP at 1 day, 1 week, and 1 month was 13.6┬▒3.7 mmHg (range: 8-24 mmHg), 13.2┬▒6.0 mmHg (range: 8-32 mmHg), and 12.4┬▒5.0 mmHg (range: 7-24 mmHg), respectively.
We also measured immediate postoperative IOP at 2 and 5 hours after the surgery in 27 eyes. In these 27 eyes, the mean preoperative IOP was 11.9┬▒2.6 mmHg (range: 7-17 mmHg) and the mean IOPs at 2 and 5 hours were 12.6┬▒5.9 mmHg (range: 5-31 mmHg) and 13.8┬▒5.1 mmHg (range: 4-29 mmHg), respectively. In 1 (3.7%) of the 27 eyes, IOP was 5 mmHg at 2 hours and 4 mmHg at 5 hours, but it was normalized to 18 mmHg at 1 day after the surgery (
Fig. 2).
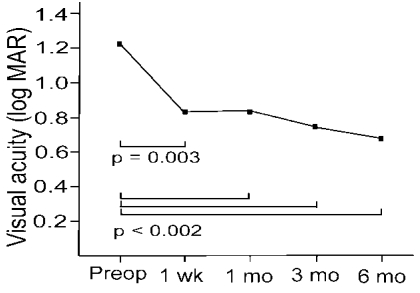
The median best corrected visual acuity (BCVA) improved from 20/400 (LogMAR, 1.21┬▒0.63) preoperatively to 20/140 (LogMAR, 0.83┬▒0.48) at 1 week (p=0.003), 20/100 (LogMAR, 0.85┬▒0.65) at 1 month (p=0.002), 20/100 (LogMAR, 0.73┬▒0.6) at 3 months (p=0.001) and 20/100 (LogMAR, 0.68┬▒0.36) at 6 months postoperatively (p<0.0001) (
Fig. 3).
During the follow-up period, no serious complications such as endophthalmitis or retinal detachment were detected. No patient developed sclerotomy-related complications. (
Table 1).
Discussion
In this study, we applied the 23-gauge TSV technique to 40 patients. Preoperative diagnoses were similar to those of previous series with 25- and 23-gauge systems including vitreous hemorrhage, diabetic macular edema, epiretinal membrane, and rhegmatogenous retinal detachment without PVR.
1-
7 Eyes with both retinal detachment and PVR, severe tractional retinal detachment or those requiring silicone oil injection were excluded, as such cases are not generally recommended for a 25-gauge system.
4-
6 Further study is therefore required to evaluate the appropriateness of using 23-gauge vitrectomy for more complicated eyes.
When performing phacovitrectomy, many surgeons using 25-gauge TSV suggest placement of microcannulas before cataract surgery to avoid introducing the microcannulas into a soft eye.
5,
8 However, we performed phacovitrectomy without preplacement of microcannulas and found no problem in introducing 23-gauge microcannulas. A preformed tunnel incision with stiletto blade may reduce the resistance of the sclera against microcannula insertion, thus preventing eyeball deformity and instability of the cataract wound. So, preplacement of the microcannula before cataract surgery may no longer be recommendable in 23-gauge TSV.
Two (5%) of 40 eyes required suturing due to leakage after removal of cannulas. Lakhanpal et al
6 reported that 5 among 140 cases which underwent 25-gauge TSV required suture placement due to leakage, and that the sutured site was near the surgeon's dominant hand, which did most of the intraocular manipulation. Because only 2 eyes required suture placement in this study, our study sample was too small to offer any support for such a trend.
In 25-gauge TSV, cases of early postoperative hypotony were firstly reported by Fujii et al.
2 O' Reilly et al.
5 reported transient hypotony in 10 of 39 cases (25.6%) and Lakhanpal et al.
6 reported that 2 out of 140 cases (1.4%) exhibited shallow choroidal detachments, which resolved in 1 week. However, there were no published data for immediate IOP changes within 1 day postoperatively in TSV. Therefore, we measured IOP at 2 and 5 hours after 23-gauge TSV in 27 eyes. Only 1 eye (3.7%) showed hypotony (IOPŌēż5 mmHg) at 2 and 5 hours after surgery, which was normalized at 1 day after the surgery. Because we observed localized blebs at the temporal side of the conjunctiva, the cause of hypotony may have been fluid leakage from the sclerotomy site. This bleb was completely resolved and IOP was normalized at postoperative day 1. No hypotony was observed at postoperative 1 day or after in any of the 40 eyes. This result is comparable to the report of Eckcardt
3 that no postoperative hypotony was observed in 41 eyes after 23-gauge TSV. However, immediate IOP data suggested that the tunnel-like incisions using stiletto blade did not completely guarantee the sealing of the sclerotomy wound. Fortunately, this transient hypotony did not show any complication during the follow-up periods. However, we need more cases and long-term follow-up to determine the effect of hypotony related with 23-gauge TSV.
Conjunctival injection and scarring were minimal with the 23-gauge system, and the external appearance was much less traumatic than that with the 20-gauge system. As in 25-gauge TSV, 23-gauge self-sealing sclerotomy obviates the need for conjunctival peritomy and suturing, and also has the advantages of the minimally invasive, sutureless vitrectomy system.
There is a concern that vitreous incarceration at the sclerotomy site might result in peripheral vitreous and retinal traction, and that the incidence of retinal detachment following 25-gauge TSV might be increased compared to that of standard 20-gauge surgery. Ibarra et al.
7 documented postoperative retinal detachment in one out of 45 consecutive eyes after 25-gauge vitrectomy and stated that the incidence of retinal detachment does not appear to be increased compared to that of standard 20-gauge surgery. Lakhanpal et al.
6 also reported no retinal detachment in their 140 consecutive cases of 25-gauge TSV. During 8.4┬▒3.4 months of follow-up, no cases of postoperative retinal detachment occurred in our series but long-term follow-up with a larger group is needed to determine the incidence of retinal detachment.
We additionally did an in vitro experiment to evaluate the efficacy of 23-gauge infusion cannula and vitreous cutter following the method as described by Fujii et al.
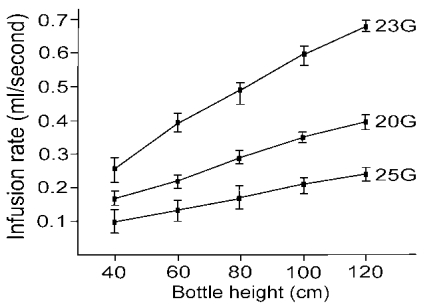
1 To compare the effectiveness of the 23- (Reusable Infusion Cannula, 0.75 mm [23 G], DORC, Zuidland, Holland), 20- (BD Infusion Cannula, 4 mm, 0.90 mm [20 G], BD, Waltham, USA) and 25-gauge (25-gauge infusion cannula, Alcon Surgical, Texas, USA) infusion cannulas, we measured the time to infuse 10ml of balanced saline solution (BSS) at a bottle height of 40, 60, 80, 100 and 120 cm. We also measured the time to aspirate 5 ml of BSS with 20- (Accurus 2500, Alcon Surgical, Texas, USA), 23- (Pneumatic Vitrectomy, 23-gauge, DORC, Zuidland, Holland) and 25-gauge (Accurus 25-gauge, Alcon Surgical, Texas, USA) ocutomes by the aspiration setting of 100, 200, 300, 400, and 500 mmHg using the same Accurus® Vitreoretinal Surgical System (Alcon Surgical, Texas, USA). Individual measurements were replicated 5 times to ensure reliability and reproducibility. In general, the average infusion rate of the 23-gauge cannula was 1.7- and 2.8-fold greater than that of the 20- and 25-gauge cannulas, respectively (
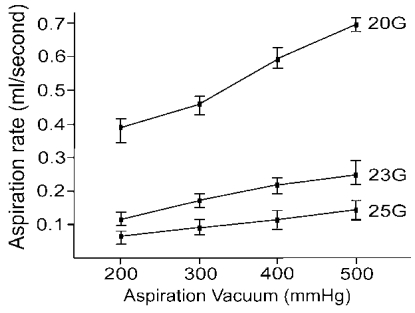
Fig. 4) and the average aspiration rate of the 20-gauge ocutome was 3.2- and 5.9-fold greater than that of the 23- and 25-gauge ocutomes, respectively (
Fig. 5). Although no controlled comparison study was performed in vivo, we can expect that the surgical time with the 23-gauge system might be at least the same as or less than that of the 25-gauge system. Although the inner diameter of 23- gauge cannula is smaller than that of 20-gauge system (0.65 mm vs. 0.80 mm), the infusion rate with the 23-gauge system was not only greater than that of the 25-gauge system, but also than that of the 20-gauge system. We speculated that there would be a bottle neck effect in 20 gauge infusion system because, there is acute narrowing of inner diameter between infusion tube and infusion tip at the joint of the flange. In 23 G system, the infusion tube is directely connected to microcannula with funnel-shaped opening which may reduce bottle neck effect. Fujii et al.
2 insisted that the 25-gauge infusion cannula should not be used in conjunction with a 20-gauge vitreous cutter, because it may result in hypotony during aspiration resulting from the functional discrepancy between the infusion and aspiration rates of both systems. We could expect that no such concern will be necessary with the 23-gauge system.
This study had some limitations. First, the relatively small sample size of this retrospective observational series and the relatively short-term follow-up were insufficient for meaningful conclusion regarding the efficacy and safety of the 23-gauge TSV. However, these promising results provide encouragement to conduct further prospective case series with a larger sample and longer follow-up. Second, we did not offer any information regarding the surgical time of 23-gauge TSV. We did not measure the precise procedure time, but, total procedure time is comparable to that of 20-gauge system. It is already known that the total procedure time of 25-gauge TSV generally was not more than that of the 20-gauge vitrectomy system in less complicated cases.
4,
9 Further study is needed to evaluate the precise surgical time depending on the step-by-step manner. Third, the surgeon's learning curve may affect the surgical results of 23-gauge TSV. Although the follow-up period was short and the sample was small, the study results confirmed a comparable surgical outcome and lack of severe complications. Considering that the 23-gauge TSV is a sturdier and more efficient instrument than the 25-gauge TSV, the effect of its learning curve may not be a major problem.
In conclusion, the 23-gauge sutureless vitrectomy technique was performed safely in cases of vitreous hemorrhage or tractional retinal detachment, epiretinal membrane, macular edema, macular hole and rhegmatogenous retinal detachment without PVR. The 23-gauge system features the potential benefits of being a sturdier and larger instrument than the 25-gauge TSV and we found that the infusion and aspiration rates were greater than those of the 25-gauge TSV system. Further study is warranted to determine if procedures required for more extensive fibrovascular proliferation can be performed with this promising 23-gauge system.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




