The increasing use of phacoemulsification, as well as the development of foldable IOLs, has allowed small incision cataract surgery.1-3 The reduction of the incision size induces less postoperative astigmatism after cataract surgery.1,4,5 The approach through a clear corneal incision, as introduced by Fine, has demonstrated increased safety, decreased inflammation and pain, as well as reduced surgically induced astigmatism.6
In addition, it is believed that a small incision induces less astigmatic change postoperatively.2,7-11 Small incision surgery shows rapid and stable optical recovery by preventing significant changes in corneal curvature. Therefore, incision size, as well as the method of nucleus removal, should be considered when comparing rehabilitation time after cataract surgery.9
In this retrospective study, we compare the induced astigmatism of temporal incisions of different sizes after phacoemulsification and posterior chamber IOL implantation.
Materials and Methods
The protocol for the research project was approved by the Ethics Committee of the Oregon Eye Institute, within which the work was undertaken, and it conformed to the provisions of the Declaration of Helsinki in 1995. Informed consent was obtained from all subjects and patient anonymity has been preserved.
We performed a nonrandomized, comparative study of the induced astigmatism of clear corneal incisions of different sizes. We reviewed the records of 121 eyes of 98 patients who underwent phacoemusification and posterior chamber IOL implantation by a single surgeon (IHF) between November 1997 and March 2001. Inclusion criteria were the absence of concurrent eye disease, no preexisting eye abnormality, and no history of eye surgery, eye trauma or systemic disease. Cases were assigned to one of three groups according to incision width. Cases of a 2.5 mm incision belonged to Group A (40 eyes), 3.0 mm incision to Group B (39 eyes), and 3.5 mm incision to Group C (42 eyes). Data for the 121 eyes were collected and analyzed for a follow-up duration of 36 months. Keratometric cylinder and axis values were collected for the three groups. Time-dependent changes in the surgically induced astigmatism (SIA) after 3 weeks, 3 months, 6 months, 9 months, 12 months, 24 months and 36 months were assessed.
The surgical procedure was performed with the preparation of a temporal, stepless, clear single-plane corneal incision under topical anesthesia. A side-port incision was made, and the anterior chamber was filled with viscoelastic material. A diamond clear cornea knife was used to form a corneal tunnel about 2.0 mm in length and 2.5, 3.0 or 3.5 mm in width. After continuous curvilinear capsulorhexis and hydro-dissection, an in situ phacoemulsification was performed using a choo-choo chop and flip technique.12 An injector system was used for in-the-bag implantation of IOLs, but in no case was it necessary to widen the original incision. The models of IOLs inserted were Alcon SA60AT and AMO Tecnics.⢠The incision was sealed with stromal hydration. At the end of the procedure, the wound was checked for leakage with fluorescein.
Data analysis
The surgically induced astigmatisms were calculated by polar value analysis.13 Astigmatisms may conveniently be symbolized as an astigmatic magnitude and direction, but are actually composed of refractive powers in the form of a polar value. Because the surgical incision in this study was made in the temporal approach at 0° or 180°, the following polar values were required:
AKP=KP(0)=M{sin2(Îą+90)-cos2(Îą+90)}
AKP(+45)=KP(45)=M{sin2(Îą+45)-cos2(Îą+45)}
(KP; polar value, M; astigmatic magnitude, Îą; direction of axis 2).
The meridional polar values express the flattening or steepening of the surgical meridian, while the polar values of the oblique meridian signify the surgically induced torque of the cylinder, according to its value. SIA (ÎAKP) expressed as a polar value is the difference between the postoperative and preoperative polar values. The Statistical Analysis System (version 6.12, SAS, Cary, NC, USA) was used for statistical analyses.
Results
A positive SIA indicates a steepening in the surgical meridian, with-the-incision or against-the-rule change. A negative SIA signifies a flattening of the surgical meridian, an against-the-incision or with-the-rule change. A positive and negative ÎAKP (+45) indicate an induced anticlockwise or clockwise torque, respectively.
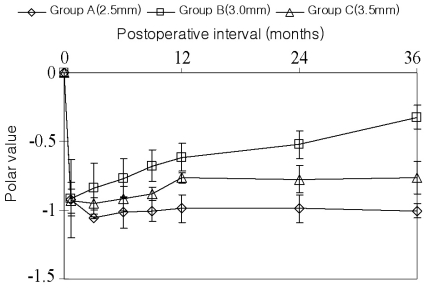
Three weeks after surgery, the SIA was -0.92 in Group A, -0.91 in Group B and -0.93 in Group C. The SIA tended to be sustained in Groups A and C, while it tended to decrease in Group B over time (Fig. 1). The difference among the groups was statistically significant after 9 months postoperatively (P<0.05) (Table 1).
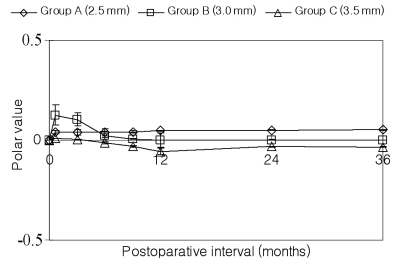
The axis deviation after surgery indicated an anticlockwise torque at 3 weeks postoperatively in all groups (Table 2). An anticlockwise deviation remained in Group A throughout the follow-up period. Group B showed less deviation at 12 months (P<0.05). Meanwhile, the torque of Group C switched to clockwise after 6 months (Fig. 2). However, there were no significant differences except at 12 months postoperatively.
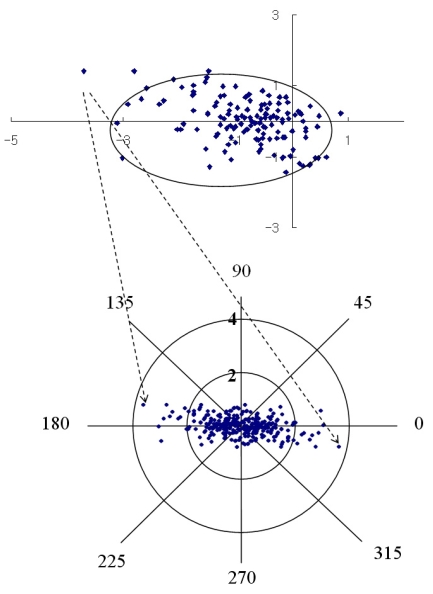
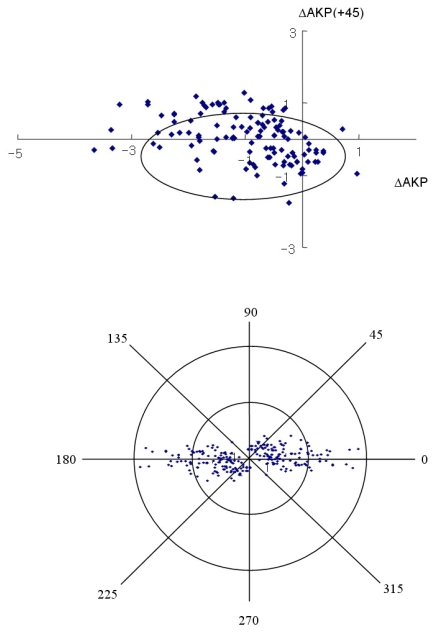
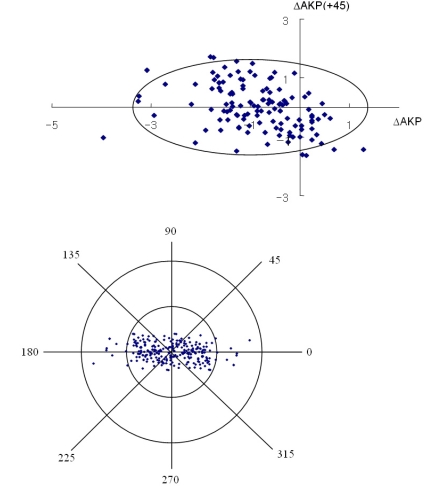
Fig. 3, 4 and 5 show the individual pairs of polar values at 12 months. It is possible to perform a point-to-point retransformation to conventional notation. Net astigmatisms are symbols, and no calculation can be performed with these entities. For each pair of polar values, there are two solutions of net astigmatisms: one in the interval from 0° to 180° rotation around origio and one depicted in the interval from 180° to 360°. When converted to conventional notation, the individual data retransformed are expressed as a 95% tolerance area looks elliptical. The 95% tolerance ellipse for SIA was smaller in Group B.
Discussion
The polar value method was used to describe the change in astigmatism following cataract surgery. The cataract incision induces variable amounts of corneal astigmatism.14 This method makes it possible to segregate magnitude and direction of the astigmatism, although the refractive change following cataract surgery has the form of a spherocylinder.15 The described polar values, AKP and AKP (+45), were used to analyze SIA following temporal incisions. Such a method yields a systemic error and supplies the correct equation for calculating average SIA, because it separates the analyses of astigmatic magnitude and direction.16 It is conceptually based on the surgically induced flattening and torque of the preoperative cylinder. Authors like to indicate astigmatic direction by the meridian rather than by the axis, because polar value analysis facilitates the understanding of induced astigmatic change.
To avoid confusion, we restricted the analysis to the astigmatic polar values AKP and AKP (+45), since the data for this study was from surgical incisions using the temporal approach. Astigmatic polar values express the change in power along the preoperatively steeper corneal meridian. Therefore, it seems preferable to use the polar value of the SIA to interpret postoperative change. A positively induced meridional polar value indicates a steepening of the surgical meridian, a negative value a flattening. A positive oblique polar value is synonymous with an anticlockwise torque, a negative value a clockwise torque.
The size, architecture, and location of the incision together influence surgically induced astigmatism. The incision is more than a port of access to the anterior chamber; it represents an important step of the operation, affecting ocular integrity and corneal stability.17 There are several benefits to using a small incision, including more rapid wound healing, less induced corneal distortion, and more control of the anterior chamber during surgery.2,4,7-9 The best incision for cataract surgery ensures no leakage and has minimal influence on corneal shape. Drews found that 2.0 mm wounds had essentially no effect on corneal astigmatism, showing almost no shift over 5 years.2
In our study, Group A did not show less induced astigmatism than the other groups. On the contrary, Group C expresses less SIA than Group A. This result suggests that a smaller incision might not always reduce the potential of an astigmatic shift. It seems that the cornea does not have the capacity necessary to tolerate true elastic deformation, so that excessive stretching may injure the cornea. Clear corneal incisions are somewhat enlarged after insertion of foldable IOLs.
Kohnen and coauthors studied the minimum incision size for IOL implantation in a cadaver eye model.5 They found that incision sizes were approximately 11% greater after the insertion of foldable IOLs. They noted tearing of Descemet's membrane and the corneal stroma at the lateral borders of the incision, especially in smaller or tighter incisions.5,18 Mamalis reported that wounds enlarged from 3% to 6% after IOL insertions.3 Therefore, the proper size of a clear corneal incision before implantation of a foldable IOL is important in preventing corneal damage by uncontrolled wound extension. Radner showed that IOL implantation through incisions that were too small intensified corneal damage with tearing of the stromal lamellae.19 It appears difficult to expand the wound at the time if IOL insertion without increasing corneal trauma.20 Naeser's polar values showed initial WTR astigmatic changes in all groups.
In our study, the values decreased gradually at subsequent follow-ups in Group B. All self-sealing incisions produce a relaxing effect in the incision axis.2,4,8,9,21,22 With absorption of fluid from the stroma, corneal flattening will diminish. However, residual WTR astigmatism may persist as a result of wound slippage.23
By reducing the time for visual stability, small incision surgery reestablishes patients' independence, returns them to the workplace quickly, and eliminates the need for several postoperative visits.9 Furthermore, minimizing corneal tissue damage minimizes postoperative complications and leads to more rapid visual recovery.
In summary, for less surgically induced astigmatism, the determination of the appropriate preimplantation wound size for insertion is important to ensure that the wound is large enough to avoid irreversible stretching, which may compromise the wound. In future research, we will measure the incision size after IOL insertion to support the findings of Group B's correlation with the less surgically induced astigmatism.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






