Triamcinolone-Induced Intraocular Pressure Elevation: Intravitreal Injection for Macular Edema and Posterior Subtenon Injection for Uveitis
Article information
Abstract
Purpose
To assess the effect of intravitreal and posterior subtenon injections of triamcinolone acetonide (TA) on intraocular pressure (IOP).
Methods
we reviewed 42 consecutive eyes after intravitreal TA injection (IVTA) and 43 eyes following posterior subtenon TA injection (PSTA). All cases had a minimum follow-up time of three months. After injection, the value and time of the maximal IOP, the amount of IOP elevation and the needs of the medication were assessed.
Results
The IOP increased significantly (p<0.001) from 16.3±2.5 mmHg preoperatively to a mean maximum of 21.7±5.3 mmHg in the IVTA group, and from 15.3±4.5 mmHg to 20.6±3.0 mmHg in the PSTA group. An elevation in the IOP of more than 5 mmHg from the baseline IOP was seen in 52.4% of the IVTA group at a mean time of 3.1 weeks postoperatively, and 44.2% of the PSTA group displayed an IOP elevation at 5.9 weeks.
Conclusions
Both developed significant elevations of IOP, but this appeared at a later date in the PSTA group. Careful follow-up after local injection of steroids is necessary.
Posterior subtenon injections of intermediate or long-acting corticosteroids are widely used as the initial treatment for visual loss caused by retinal edema or other reversible effects of inflammation in patients with intermediate uveitis.1 A subtenon injection delivers a large amount of the drug to the posterior segment of the eye via transscleral absorption, and it also minimizes the risk of systemic side effects.2,3
Recently, intravitreal injections of triamcinolone acetonide (IVTA) have been widely used as an effective method in the treatment of conditions such as uveitis and macular edema that are secondary to retinal vascular disease. IVTA has also been used to treat intraocular proliferations such as proliferative vitreoretinopathy and choroidal neovascularization resulting from age-related macular degeneration.4-7
In comparison with other corticosteroids, triamcinolone acetonide (TA) is a minimally water-soluble steroid in a suspension form; as a result, it can maintain a long-term intraocular concentration for an expanded period of time. TA has been reported to be present in the eye for as long as six months after the injection, and recent studies have found it to be present in measurable concentrations up to 1.5 years after IVTA 20-25 mg.8
Because both topical and systemic corticosteroids are known to be associated with an increase in intraocular pressure (IOP),9 this extended intraocular presence of TA may lead not only to secondary ocular hypertension but also to steroid-induced secondary open-angle glaucoma necessitating trabeculectomy.10
The purpose of this study was to assess the effects of TA when delivered into the vitreous or posterior subtenon space on the IOP.
Materials and Methods
A retrospective chart review was performed to identify patients who had received intravitreal or posterior subtenon injections of TA for the treatment of chronic macular edema, choroidal neovascularization, or uveitis. Patients also had to have had at least three months of follow-up from January 2000 to December 2003. They were excluded if they had had a previous intraocular surgery, acute inflammation in the anterior chamber, an IOP value higher than 22 mmHg, or a history of glaucoma. A total of 85 eyes in 66 patients met the inclusion criteria and were eligible for the study.
One group, made up of patients in which TA had been injected intravitreally (the IVTA group), included 42 consecutive eyes of 41 patients. Twenty-one eyes in 20 men and 21 eyes in 21 women were included. The mean age of the patients was 58.5 years (range, 39-75 years). Indications were diabetic macular edema (24 eyes), retinal vascular occlusive disease (eight eyes), uveitis (five eyes), and exudative AMD (five eyes).
In the other group, TA was injected into the posterior subtenon space (PSTA group). This group consisted of 43 consecutive eyes in 25 patients. Twenty-eight eyes in 16 men and 15 eyes in 9 women were included. The mean age was 48.3 years (range, 33-80 years). Most patients were diagnosed with idiopathic intermediate uveitis, with the exception that two eyes had Behcet and Harada Disease.
The intravitreal injections of TA were carried out in an office setting. Drops of proparacaine hydrochloride 0.5% (Alcaine, Alcon. Inc.) and ofloxacin 0.3% were administered to the affected eyes. Then the eyelids and surrounding areas were scrubbed with Povidone-iodine 5% and an eyelid speculum was placed. TA (40 mg/ml, Dong-Kwang Pharmaceutical Company) 4 mg in 0.1 ml was injected into the vitreous cavity through the inferotemporal pars plana, 3.5 mm posterior from the limbus in the phakic eyes.
After injection, patients were asked to maintain a sitting position. The anterior and posterior segments and the IOP were checked two hours after the injection.
For the posterior subtenon injection, TA 40 mg (1 ml) was administered into the inferotemporal or inferonasal posterior fornix through a 5/8-inch, 26-gauge needle attached to a tuberculin syringe. All patients were prescribed ofloxacin 0.3% eye drops four times daily for seven days.
The applanation tonometry measurements, slit lamp examination and fundus examination for both the injected and fellow eyes were recorded on the day of injection and one day, one week, two weeks, and one month after the injection, and thereafter as dictated by the clinical response. The data were analyzed using a two-sample t-test for independent samples that are not distributed normally.
The pre-injection IOP was statistically compared with the post-injection IOP. The statistical analyses of the differences between the mean pre-injection pressure and post-injection pressure during the follow-up periods were performed with a two-tailed paired t-test using the statistical software program SPSS version 11.5. The effects of diabetes, hypertension, age and refractive errors on IOP and the change of IOP after injection were also analyzed with a Spearman rank correlation coefficient using SPSS version 11.5.
Results
In the IVTA group, the difference between the mean pre-injection pressure (16.3±2.5 mmHg, n=42 eyes) and the maximum post-injection pressure (21.7±5.3 mmHg, n=42 eyes) that occurred after 10.2±11.5 weeks (mean time) was statistically significant (p<0.001, paired two-tailed t-test) (Table 1).
In the PSTA group, the difference between the mean pre-injection pressure (15.3±4.5 mmHg, n=43 eyes) and the maximum post-injection pressure (20.6±3.0 mmHg, n=43 eyes), which occurred after 12.6±15.1 weeks (mean time), was also statistically significant (P<0.001, paired two-tailed t test) (Table 1).
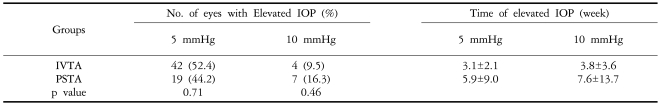
Twenty-three out of 42 eyes (52.4%) in the IVTA group had an IOP increase of 5 mmHg or greater. The mean time for a 5-mmHg or greater increase in IOP to occur was 3.1 weeks [standard deviation (SD)=2.1 weeks]. Nineteen out of 43 eyes (44.2%) in the PSTA group demonstrated an IOP increase of 5 mmHg or greater. The mean time for an increase in IOP of 5 mmHg or more to occur was 5.9 weeks (SD=9.0 weeks). The difference between the two groups was not statistically significant (p=0.71) (Table 2).
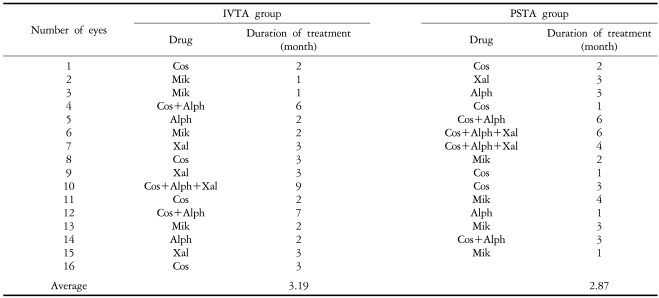
Four out of 42 eyes (9.5%) had an increase in IOP of 10 mmHg or greater at a mean of 3.8 weeks (SD=3.6) in the IVTA group, and 7 of 43 eyes (16.3%) at a mean of 7.6 weeks (SD=13.7) in the PSTA group also had an IOP increase of 10 mmHg or greater. The difference was not statistically significant (P=0.46) (Table 2). Sixteen eyes (35.7%) from the IVTA group and 15 eyes (34.9%) in the PSTA group were found to have an IOP of 22 mmHg or higher, and they were treated with IOP-lowering eye drops. The mean duration of medication was 3.2 months in the IVTA group and 2.9 months in the PSTA group (Table 3, 4).

Required medications for elevated intraocular pressure after triamcinolone acetonide injection during the follow-up period
The elevated IOP was successfully controlled with IOP-lowering eye drops in most patients, except for one eye (2.4%) in the IVTA group and two eyes (4.7%) in the PSTA group that had undergone a trabeculectomy. Most of the topical anti-glaucoma medications were successfully discontinued 12 months postoperatively.
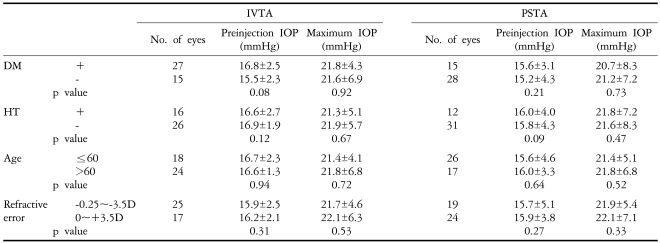
The presence of diabetes mellitus or hypertension, age and refractive errors (-3.5D~+3.5D) had no influence on the change of the IOP in either group (p>0.05) (Table 5). Other severe ocular complications such as vitreous hemorrhage, cataracts, retinal detachment, or endophthalmitis were not observed in either of the two groups.
Discussion
Intraocular neovascular diseases such as exudative AMD and proliferative diabetic retinopathy are one of the leading causes of acquired vision loss. In addition, edematous diseases of the retina such as diffuse diabetic macular edema and persistent cystoid macular edema also significantly contribute to the incidence of acquired vision loss. Steroids can be used for these diseases because they reduce vascular leakage and inhibit intraocular cell proliferations.4-7 Both systemic and local administration methods of the steroids have been used to deliver the drug, but systemic administration has limitations because of its various systemic side effects and poor penetration into the eye. The local administration of steroids such as subtenon or intravitreal injections reduces these limitations and maintains effective intraocular concentrations.
The most common complication of local or systemic steroids is the elevation of IOP. Corticosteroid-induced ocular hypertension is a well-known phenomenon. It has been speculated that a genetic difference exists between corticosteroid responders and nonresponders, and that patients who show an increase in IOP have a different or a more sensitive corticosteroid receptor. The mechanism of elevated IOP due to steroids has been thought to be secondary to an increased resistance to aqueous outflow. This event may be induced by the accumulation of polymerized glycosaminoglycans in the trabecular meshwork, an increased expression of collagen, elastin, or fibronectin in the extracellular matrix, a decrease in the expression of extracellular proteinases, the suppression of phagocytosis by the trabecular endothelium, and a reorganization of the trabecular meshwork cell cytoskeleton into cross-linked actin networks.11
TA is commonly used due to its longer half-life (1.6 days) than that of dexamethasone (2.5 hours); its minimal water solubility also contributes to its longer duration of action. However, the longer duration of action also increases the incidence of ocular hypertension.
Using indirect ophthalmoscopy and scleral depression, Sophie et al.13 noted the presence of TA crystals in the vitreous for at least 12 weeks. In contrast, Scholes et al.13 reported a marked change in the appearance of what was thought to be TA, and they were unable to quantify the steroid using indirect ophthalmoscopy.
Beer et al.14 determined that measurable concentrations of TA would be expected to last for approximately three months (93±28 days) in the non-vitrectomized eyes. Although the intraocular concentration of TA may fall below the therapeutic range in different clinical settings well before 90 days have passed, the persistence of even a trace amount may be related to the prolonged ocular hypertension occasionally seen in patients. It was also reported that the elevated IOP levels associated with IVTA that were not controlled with maximal anti-glaucoma medication could effectively be treated through a vitrectomy-assisted removal of the TA.15
The incidence of elevated IOP in eyes receiving IVTA was high and ranged from 20-52%.16-18 Sophie et al.12 found that 21 of 43 eyes (48.8%) demonstrated an increase in IOP of 5 mmHg or greater, and 12 of 43 eyes (27.9%) had an IOP increase of 10 mmHg or greater after a 4-mg intravitreal TA injection.
The ranges of our IOP increases were from 3 to 22 mmHg in the IVTA group and from 2 to 18 mmHg in the PSTA group. We classified the increase in IOP into either the 5 mmHg or greater category or the 10 mmHg or greater category in our disposition. Our study showed a similar incidence of increase in IOP in both categories, so that 23 of 43 eyes (52.4%) had an increase in IOP of 5 mmHg or greater, and 4 of 43 eyes (9.5%) had an IOP increase of 10 mmHg or greater. Yang et al. noted that the IOP in IVTA is more elevated at a younger age (<60 years),19 but our study showed no relationship between the elevation of IOP and the patient's age (Table 5).
The subtenon route is routinely used for the administration of steroids to the posterior segment, but the need to diffuse across the sclera and the vascular bed in the choroid coupled with the highly variable rate of steroid dissolution from crystals make intraocular steroid levels variable and difficult to assess and to adjust. Kalina et al.20 reported that pharmacologically-active triamcinolone was identified for up to 13 months following subconjunctival injection (with a range from 3 to 13 months). Medically-unresponsive IOP elevation may occur as late as six months after a periocular triamcinolone injection or the surgical excision of a subconjunctival deposit.
The onset of pressure increase in the PSTA group was noted at a mean of 7.1 weeks (with a range of 1.5 to 16 weeks) and lasted a mean duration of three months (with a range of one to five months).21 Glaucoma secondary to periocular repository corticosteroids tends to be of a delayed onset and a long duration.21,22 However, the role of posterior subtenon corticosteroids in ocular hypertension is not always clear because the increased intraocular pressure may be a result of the disease itself or it could be influenced by the effects of corticosteroids.
It was reported that 12% of patients with acute inflammation and 27% of patients with chronic inflammation had elevated IOP levels.23 However, Kim et al.24 determined that the true elevation percentage of IOP due to inflammation is 12% in acute inflammation because many factors (such as PAS etc.) can influence the elevation of IOP in the presence of chronic inflammation. Because we excluded patients who had acute inflammation in the anterior chamber before injection, true cases of elevated IOP with inflammation were not included. Therefore, the elevation of IOP in the PSTA group seems to be an effect of the TA itself.
We also found that the increases in IOP of both 5 mmHg and 10 mmHg or greater appeared later in the PSTA group than in the IVTA group, but the amount of elevated IOP and duration of anti-glaucoma medication needed were similar in both groups. One eye (2.4 %) of 42 eyes in the IVTA group and two eyes (4.7 %) of 43 eyes in the PSTA group received a trabeculectomy due to medically uncontrolled glaucoma.
IOP-lowering eye drops were not used in patients whose IOP was 21 mmHg or less, even if they showed an increase in IOP of 5 mmHg or greater postoperatively.
While most patients who had elevated IOP were well-controlled with IOP-lowering medications, some of them needed surgical treatment. A careful follow-up of IOP levels should be performed in all patients who have received periocular and intravitreal steroid injections.
The small number of patients in the study group is one of the limitations of our study. Prospective studies of a larger study group are necessary to better demonstrate the effect of periocular and intravitreal steroid injections on IOP.
Notes
This study was presented in part at the Korean Ophthalmological Society Spring Meeting, Busan, Korea, in April 2004.