Vitreous Opacity Following Intravitreal Brolucizumab Injection: A Case Series Review
Article information
Abstract
Purpose
To investigate cases of vitreous opacity (VO) similar to asteroid hyalosis (AH) after intravitreal brolucizumab injection.
Methods
A retrospective chart review was conducted to identify cases showing VO similar to AH among patients who received intravitreal brolucizumab injections at our retinal clinic from January 2022 to January 2023.
Results
A total of 220 brolucizumab injections were administered at our hospital. VO, showing yellow-white brilliant reflective particles, was found in six patients (2.7%). When VO occurred, all patients complained of floaters, although none of them complained of other symptoms including decreased visual acuity, pain, or conjunctival redness. The mean number of brolucizumab injections was 2.57 ± 2.38. No significant visual impairment was observed while VO was present. VO improved in all cases, and four cases improved without any treatment. The mean interval from onset to disappearance of VO was 8.0 ± 3.1 weeks.
Conclusions
VO, similar to AH, can occur with a relatively high probability after intravitreal brolucizumab injections. Patients complained of severe floaters, but VO was not accompanied by other symptoms including vision impairment, injection, and pain. The VO disappeared after approximately 4 to 14 weeks. In case that other inflammatory findings are not severe, close follow-up without treatment may be sufficient. If a patient complains of floaters after an intravitreal brolucizumab injection, close fundus observation is necessary to evaluate the VO.
Age-related macular degeneration (AMD) is a prevalent, chronic, and progressive retinal degenerative disease of the macula that affects the elderly and causes central vision impairment [1]. Various anti–vascular endothelial growth factor (anti-VEGF) agents have been developed to treat AMD. Pharmacological treatment of neovascular AMD was initiated using pegaptanib as an anti-VEGF drug; however, it was then revolutionized by the introduction of newer anti-VEGF agents such as ranibizumab (Lucentis, Genentech) and aflibercept (Eylea, Regeneron). Brolucizumab (Beovu, Novartis Pharma AG), a new anti-VEGF agent, was approved by the US Food and Drug Administration (FDA) in late 2019 for the treatment of wet AMD. Brolucizumab is a low molecular weight humanized single- chain variable antibody fragment VEGF inhibitor [2]. This 26-kDa humanized antibody is half the size of ranibizumab and may have a relatively easier manufacturing process; it has been suggested to show more tissue penetration, with 2.2- and 1.7-fold higher exposure in the neurosensory retina and retinal pigment epithelium, respectively, due to its smaller size [3–5].
However, various adverse effects of brolucizumab have also been reported. The FDA has reported a 4% probability of intraocular inflammation (IOI) and a 1% probability of retinal artery occlusion in patients treated with this drug [6]. Baumal et al. [7] reported that retinal vasculitis and IOI are mostly diagnosed 30 days after the most recent brolucizumab injection. They showed that the affected eyes developed focal or elongated segmental sheathing, vascular nonperfusion, sclerotic vessels, cotton-wool spots, irregular and dilated veins, perivenular hemorrhage, and phlebitis foci. Immediate hypersensitivity reactions, similar to toxic anterior segment syndrome, can also occur after intravitreal injection of brolucizumab [8]. Other reported adverse effects include lenticular opacities, punctate keratitis, corneal abrasion, posterior capsule opacification, cataracts, increased intraocular pressure, blepharitis, conjunctivitis, and iritis [9]. Meanwhile, Yoshikawa et al. [10] reported a case of brolucizumab-associated inflammation in the vitreous cavity, manifesting as a black smoke-like shadow on optical coherence tomography (OCT) examination. In our retinal clinic, we encountered several cases of vitreous opacity (VO) after intravitreal brolucizumab injection, with an appearance similar to that of asteroid hyalosis (AH). All patients complained of sever floaters.
Here, we report six cases of VO with floaters, which have been rarely reported previously, among patients who had been treated with brolucizumab.
Materials and Methods
This study was was approved by the Institutional Review Board of Konyang University Hospital (No.2023-11-005). The requirement for informed consent was waived due to the retrospective nature of the study. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
A retrospective chart review was conducted to identify cases showing VO among patients who received intravitreal brolucizumab (Beovu) injections at our retinal clinic (Konyang University Hospital, Daejeon, Korea) from January 2022 to January 2023. Each patient had a history of treatment with different types of injections. One retinal specialist (MWL) confirmed the occurrence and improvement of VO after intravitreal brolucizumab injection by examining the fundus using a slit-lamp microscope and wide-field fundus photography. All patients underwent a slit-lamp examination, fundus examination, best-corrected visual acuity (BCVA) measurement, intraocular pressure measurement, fundus photography, and OCT. The severity of inflammation in the anterior chamber was described on the basis of the standardized uveitis nomenclature working group grading system [11]. Aseptic disinfection was performed using povidone-iodine before all injections. The meibomian gland was washed using a cotton swab soaked in povidone-iodine, and the conjunctiva was washed for 30 seconds using a povidone-iodine solution after inserting the speculum. Brolucizumab was prepared in unit-dose vials obtained directly from the manufacturer. The intravitreal injections were administered by a single ophthalmologist.
Results
During the study period, our hospital administered a total of 220 intravitreal brolucizumab injections to 39 patients. VO similar to AH was found in six patients (2.7%) who received intravitreal brolucizumab injections; none of these patients had a history of hypertension, while two of them had a history of diabetes. In addition, none of these patients had a systemic autoimmune disease or a history of ophthalmic disease except for AMD. Five eyes were diagnosed as showing exudative AMD and one was diagnosed as showing polypoidal choroidal vasculopathy (Table 1). When VO occurred, all patients complained of floaters, although none of them complained of decreased visual acuity. No patient complained of pain or conjunctival redness. The mean central macular thickness (CMT) was 318.0 ± 166.9 μm, and VO improved in all cases. The average period until VO occurred after intravitreal brolucizumab injection was 4.3 ± 1.9 weeks, and the average number of injection until vitreous opacity occurred was 3.0 ± 1.9 times. The mean interval from onset to disappearance of VO was 8.0 ± 3.1 weeks. The mean number of brolucizumab injections was 2.57 ± 2.38. There was no case of re-injecting brolucizumab into a patient who developed vitreous opacities after intravitreal brolucizumab injection.
Case 1
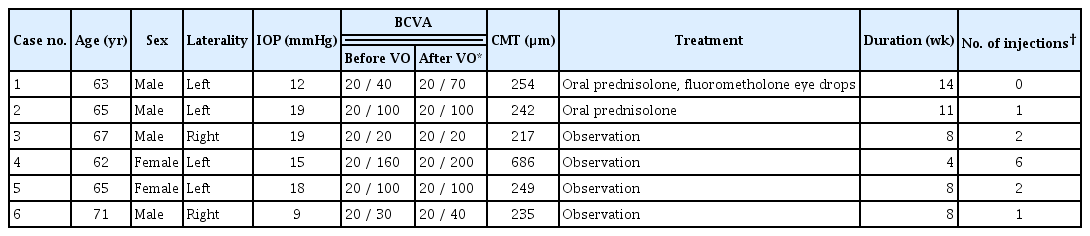
A 63-year-old man with polypoidal choroidal vasculopathy in the left eye received 14 intravitreal aflibercept injections. The patient had a history of diabetes, but there was no past history of uveitis or autoimmune disease. While the patient showed improvements until the 12th injection, no improvement in subretinal fluid (SRF) was observed after the 13th intravitreal aflibercept injection. Therefore, aflibercept was replaced with brolucizumab. The BCVA before brolucizumab injection was 20 / 40; CMT was 302 μm; and small amounts of SRF and intraretinal fluid (IRF) were observed in the OCT examination. VO occurred 4 weeks after the first injection of brolucizumab, and the patient complained of floaters. The BCVA was 20 / 70 at the time of the occurrence of VO. On OCT examination, CMT was 254 μm, and although fibrovascular pigment epithelial detachment (PED) and SRF showed improvements, mild VO was observed. Slit-lamp examination showed a cell score of 0.5+ in the anterior chamber. The VO was similar to AH, showing yellow-white brilliant reflective particles with a severity of 1+ (Fig. 1A–1C). No prominent inflammatory findings were observed on fluorescein angiography. The patient was prescribed 15 mg of prednisolone for 2 weeks, which was stopped after reducing the dose by 5 mg every week. The patient also received fluorometholone eye drops four times a day for 1 week. VO began to improve 2 weeks after the treatment and completely disappeared after 14 weeks. Subsequently, the patient’s symptoms also improved. After the VO improved, BCVA was 20 / 40 and CMT was 241 μm. OCT examinations did not show SRF and VO, and only fibrovascular PED, similar to that previously observed, was noted.
Images for case 1. The patient was a 63-year-old man with polypoidal choroidal vasculopathy in the left eye. After 14 intravitreal aflibercept injections, the drug was replaced with brolucizumab. Vitreous opacities were observed 4 weeks after the first brolucizumab injection. (A) Color fundus photographs vitreous opacity particles (arrows). (B) Optical coherence tomography showing pigment epithelial detachment and vitreous opacities (arrows). (C) Vitreous opacity showing yellow-white brilliant reflective particles (arrow) was observed using an ophthalmoscopic contact lens.
Case 2
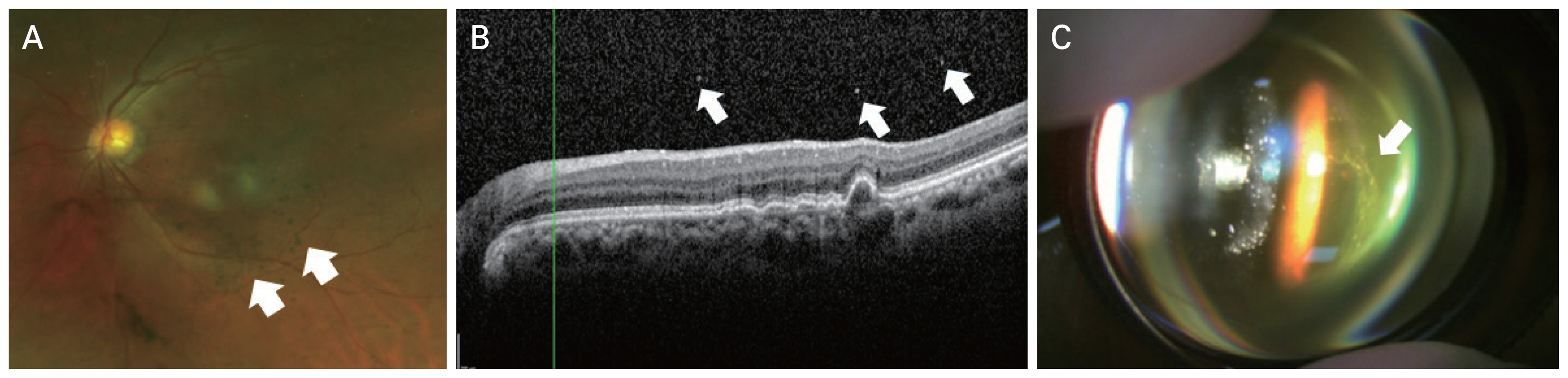
A 65-year-old man with exudative macular degeneration in the left eye received 24 intravitreal aflibercept injections. The patient had a history of diabetes, but there was no past history of uveitis or autoimmune disease. The SRF and PED did not improve despite repeated aflibercept injections. Therefore, aflibercept was replaced with brolucizumab. The SRF decreased after the first injection of brolucizumab. Two weeks after the second brolucizumab injection, the patient visited the hospital complaining of floaters. The BCVA before the occurrence of VO was 20 / 100 and CMT was 257 μm. Slit-lamp examination revealed no inflammation in the anterior chamber or anterior vitreous, but VO was observed near the macula. After the occurrence of VO, BCVA was 20 / 100 and CMT was 242 μm. OCT examinations did not show SRF and IRF, but only PED similar to that identified previously was observed. VO was similar to AH, showing yellow-white brilliant reflective particles (Fig. 2A, 2B). The VO severity was 1+. No noticeable inflammatory findings were observed on fluorescein angiography. The patient was treated with 20 mg of prednisolone for 2 weeks, which was discontinued after reducing the dose by 5 mg every week. VO began to improve after 1 week and completely disappeared after 11 weeks. After the VO improved, the BCVA was 20 / 100, and the CMT was 270 μm. On OCT examination, a small amount of SRF remained and fibrovascular PED was observed.
Images for case 2. The patient was a 65-year-old man with exudative macular degeneration in the left eye who received the first intravitreal brolucizumab injection after 24 aflibercept injections over 1 month. Although the subretinal fluid decreased, vitreous opacities were observed 2 weeks after the second brolucizumab injection. (A) Color fundus photograph showing brilliant yellow particles (arrows). (B) Optical coherence tomography showing vitreous opacities (arrows) and focal outer retinal atrophy.
Case 3
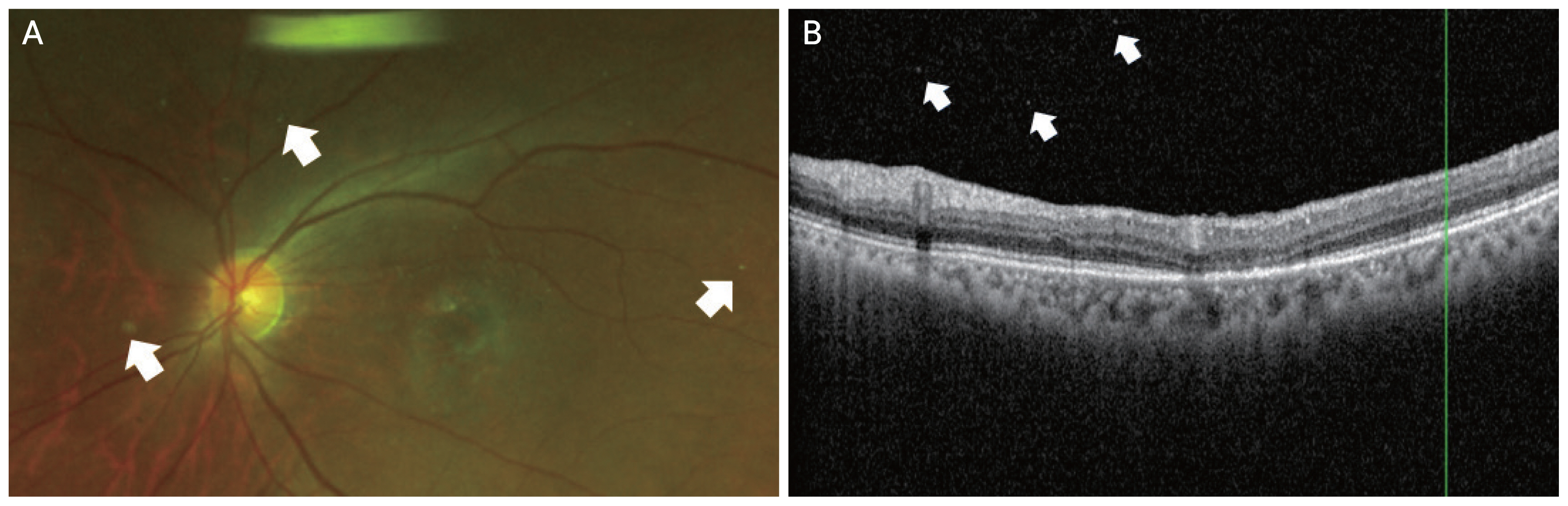
A 67-year-old man with exudative AMD of the right eye had been receiving intravitreal injections at our hospital since 2019. The patient had no previous medical history such as hypertension or diabetes, and there was no history of uveitis or autoimmune disease. The patient had received nine intravitreal bevacizumab injections and 12 intravitreal aflibercept injections in the right eye. SRF persisted even after the last aflibercept injection; therefore, aflibercept was replaced with brolucizumab. SRF decreased slightly after the first injection of brolucizumab. Three weeks after the third brolucizumab injection, the patient visited our hospital complaining of floaters. He developed VO in the right eye 2 months after the third intravitreal brolucizumab injection. Before VO occurred, BCVA of the right eye was 20 / 20 and CMT was 217 μm. On OCT examination, a small amount of remnant SRF and fibrovascular PED were observed. VO occurred 8 weeks after the third brolucizumab injection. The BCVA after the occurrence of VO was 20 / 20. Anterior chamber or anterior vitreous inflammation was not observed. The VO resembled AH, showing whitish particles (Fig. 3A, 3B). The severity of VO was 1+. After consulting with the patient, we decided to observe him without any special treatment. The VO began to improve after 4 weeks. It completely disappeared after 8 weeks despite the absence of specific treatment. After the VO disappeared, BCVA in the right eye was 20 / 20. On OCT, SRF and VO were not observed, and only fibrovascular PED, similar to that previously observed, was observed.

Images for case 3. A 67-year-old man with exudative age-related macular degeneration of the right eye had been receiving intravitreal injections at our hospital since 2019. Vitreous opacities were observed 3 weeks after the third brolucizumab injection. (A) Color fundus photograph showing whitish brilliant reflective particles (arrows). (B) Optical coherence tomography showing multiple vitreous opacities (arrows).
Case 4
A 62-year-old woman with exudative AMD of the left eye was treated with several intravitreal anti-VEGF injection at our hospital from 2016. The patient had no previous medical history such as hypertension or diabetes, and there was no history of uveitis or autoimmune disease. The patient had received seven intravitreal bevacizumab injections, 15 intravitreal aflibercept injections, and five intravitreal ranibizumab injections. The last injection before replacement was administered with ranibizumab; however, the patient’s IRF deteriorated. Therefore, brolucizumab was used instead. VO occurred within 4 weeks after the seventh brolucizumab injection. Before the occurrence of VO, BCVA in the left eye was 20 / 160. OCT revealed mild IRF and fibrovascular PED. After the occurrence of VO, the BCVA of the left eye was 20 / 200. Anterior chamber or anterior vitreous inflammation was not observed. The VO resembled AH (Fig. 4A, 4B). The severity of the VO was 0.5+, and the CMT was 686 μm. After the consultation, the patient was observed without treatment. The VO began to improve after 2 weeks. The VO completely disappeared after 4 weeks without any special treatment. After the VO improved, the BCVA of the left eye was 20 / 200, and the CMT was 297 μm. On OCT, mild IRF and fibrovascular PED similar to those of the previous examination were observed; however, VO and SRF were not observed.

Images for case 4. A 62-year-old woman with exudative age-related macular degeneration of the left eye was treated with several intravitreal anti–vascular endothelial growth factor injections at our hospital since 2016. Vitreous opacities were observed 4 weeks after the seventh brolucizumab injection. (A) Color fundus photograph showing yellowish brilliant reflective particles (arrows). (B) Optical coherence tomography image showing vitreous opacities (arrows) and outer retinal atrophy.
Case 5
A 65-year-old woman with exudative macular degeneration in the left eye was treated with one intravitreal bevacizumab injection, six intravitreal aflibercept injections, and 10 intravitreal ranibizumab injections at our hospital. The patient had no previous medical history such as hypertension or diabetes, and there was no history of uveitis or autoimmune disease. After the 10th ranibizumab injection, the SRF in the left eye increased, and ranibizumab was replaced with brolucizumab. The SRF decreased after the first brolucizumab injection. Four weeks after the third brolucizumab injection, the patient visited our hospital complaining of floaters. No anterior inflammation was observed. VO was observed. BCVA was 20 / 100 before the third brolucizumab injection, while it was 20 / 100 and CMT was 249 μm after VO occurred. The VO was similar to AH with white spherical particles and a severity of 1+ (Fig. 5A, 5B). VO improved within 8 weeks without any treatment. After the VO improved, the BCVA was 20 / 100 and CMT was 253 μm. On OCT examination, a small amount of SRF remained and fibrovascular PED was observed.
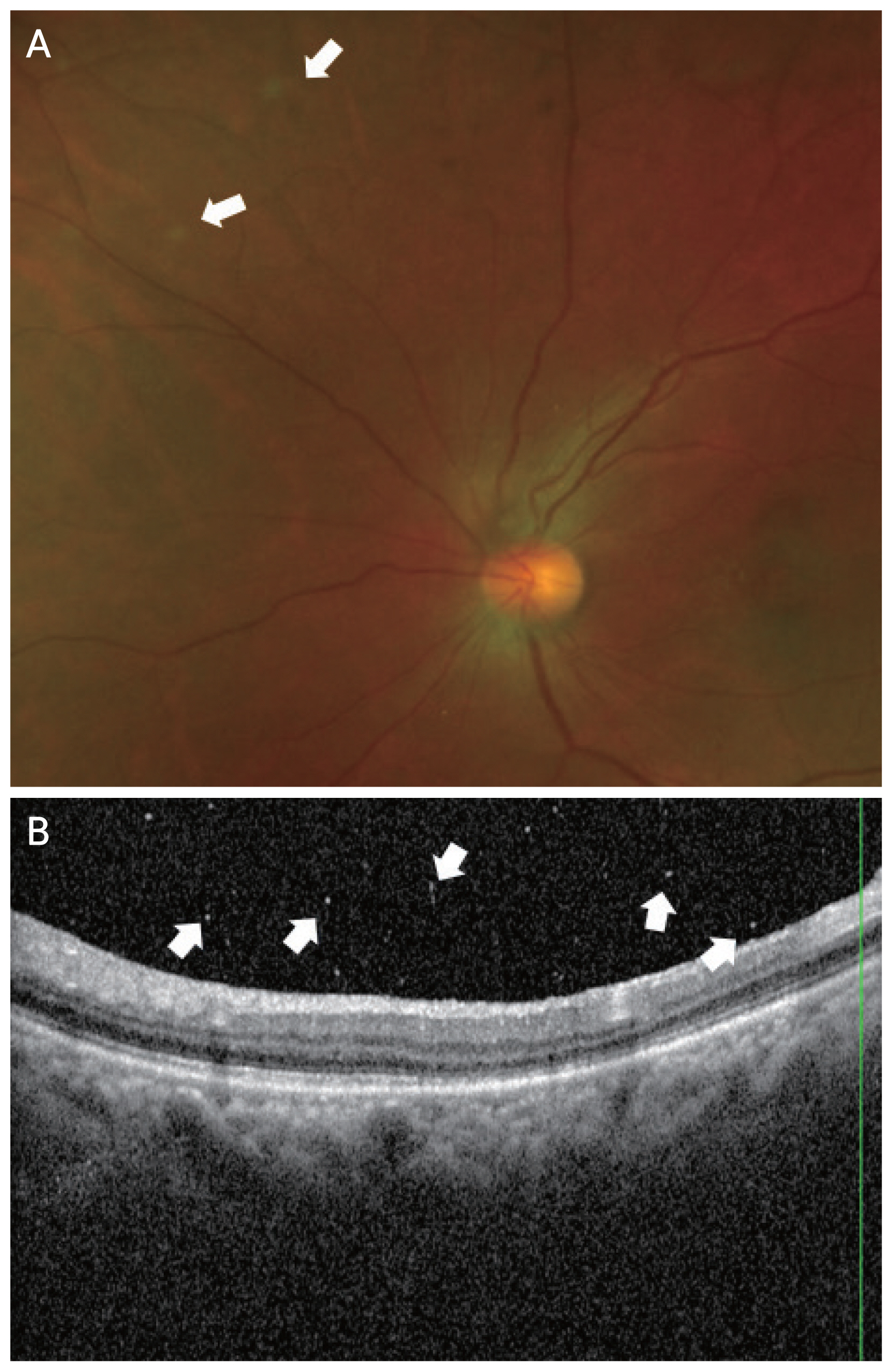
Images for case 5. A 65-year-old woman with exudative macular degeneration in the left eye was treated with various anti–vascular endothelial growth factor injections at our hospital. Vitreous opacities were observed 4 weeks after the third brolucizumab injection. (A) Color fundus photography showing white spherical particles (arrows). (B) Optical coherence tomography showed several vitreous opacities (arrows).
Case 6
A 71-year-old man showing exudative macular degeneration in the right eye was treated with 17 intravitreal injections at our hospital. The patient had no previous medical history such as hypertension or diabetes, and there was no history of uveitis or autoimmune disease. After the third aflibercept injection, PED and SRF persisted, and aflibercept was replaced with brolucizumab. The BCVA before the occurrence of VO was 20 / 30. VO occurred 4 weeks after the second injection of brolucizumab. After VO occurred, the BCVA was 20 / 40 and CMT was 235 μm. Anterior chamber or anterior vitreous inflammation were not observed. The VO resembled AH, showing bright yellow round particles (Fig. 6A–6C). The VO severity was 1+. OCT revealed VO, mild SRF, and fibrovascular PED. From week 4 after the onset of VO, the symptoms of floaters gradually began to decrease. The VO improved after 8 weeks without any treatment. After improvement in VO, the BCVA was 20 / 30 and CMT was 389 μm. On OCT examination, the PED had deteriorated; therefore, brolucizumab was replaced with aflibercept.
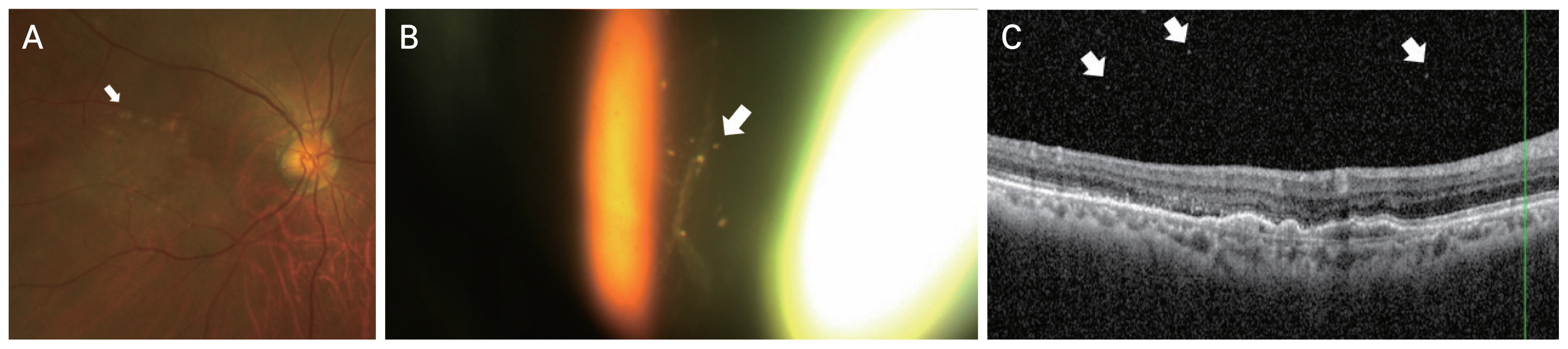
Images for case 6. A 71-year-old man with exudative macular degeneration in the right eye was treated with 17 intravitreal injections at our hospital. After the third aflibercept injection, it was replaced with brolucizumab, and vitreous opacities were observed 4 weeks after the second brolucizumab injection. (A) Color fundus photography revealed bright yellow round particles (arrow). (B) Optical coherence tomography showed fibrovascular pigment epithelial detachment, subretinal fluid, and vitreous opacity (arrow). (C) Vitreous opacity showing yellow-white brilliant reflective particles (arrows) were observed using an ophthalmoscopic contact lens.
Discussion
The adverse reactions caused by intravitreal brolucizumab injections, such as IOI, retinal artery occlusion, and iritis, have been reported in previous studies [2,7,9]. However, adverse reactions associated with VO have rarely been reported. At our retinal clinic, VO after intravitreal brolucizumab injection occurred in six of 220 injections, indicating a relatively high probability of 2.6%. In all cases, VO appeared as yellow-white brilliant reflective particles on fundus examination. All VO cases required an average of 2.8 weeks from the onset to resolution of VO. VO completely improved without any specific treatment in four cases.
Noninfectious endophthalmitis or vitritis can be accompanied by floaters after intravitreal anti-VEGF injections of bevacizumab, aflibercept, and ranibizumab [12]. Williams et al. [12] reported that the rates of noninfectious vitritis per injection were 0.10%, 0.02%, and 0.16% for bevacizumab, ranibizumab, and aflibercept, respectively. Goldberg et al. [13] reported 20 cases of VO among 5,356 aflibercept injections that showed mild to severe VO. However, few reports have described VO without other complications such as vasculitis after brolucizumab injection. A total of six cases (2.7%) of VO accompanied by floaters were observed after 220 intravitreal injections at our retinal clinic, which showed a higher incidence rate than other anti-VEGF agents. Various types of intraocular inflammation caused by brolucizumab have been reported, and VO may be a common complication. Additional multicenter studies are required in the future.
The mechanism by which VO may occur after intravitreal anti-VEGF injection has been postulated to involve specific antibodies to the drug in patients, a history of previous uveitis, autoimmune diseases, agitation of the syringe, and potential contamination with impurities or bacterial toxins [14]. The inflammation associated with these factors may be also correlated with brolucizumab. Notably, VO seems to occur more frequently with brolucizumab than with other anti-VEGF agents, possibly due to the higher dosage related to its low molecular weight compared to other drugs [15]. Further research is warranted to elucidate the underlying mechanisms and establish a comprehensive understanding of these observations.
Kaya et al. [16] reported an IOI that occurred after injection of aflibercept and ranibizumab. At the time of occurrence of IOI, all patients complained of decreased visual acuity, and 38.5% complained of floaters. Pilli et al. [17] reported a case in which the visual acuity decreased due to vitreous inflammation after a second intravitreal bevacizumab injection. In our study, the patients did not complain of symptoms such as visual impairment or pain. A visual acuity test performed at our retinal clinic did not reveal a significant decrease. Anterior chamber inflammation was rarely observed, and fluorescein angiography showed no definite signs of inflammation. VO, which resembles AH caused by brolucizumab injection, was not accompanied by severe inflammation, and the typical symptoms seen in IOI, such as impaired visual acuity, pain, and injection, were not present. However, all patients complained of severe floaters even though the VO was mild. Therefore, a detailed fundus examination and careful observation are necessary if a patient complains of floaters after intravitreal brolucizumab injection.
AH, previously called Benson disease, is characterized by small, yellow, and white particles in the vitreous cavity [18]. Despite often obscuring a clear view of the fundus, AH rarely reduces the patient’s visual acuity. This is because the surface of AH fragments is smoother than that of vitreous collagen aggregates [19]. Several studies have reported an association between AH and systemic diseases, such as diabetes, hypercholesterolemia, hypertension, and hypercalcemia [20–24]. In 1921, Verhoeff [25] proposed that asteroid bodies are fatty acid carboxylates bonded to calcium. On OCT examination, asteroid bodies appear as hyperreflective materials in the vitreous cavity [26]. In our study, despite differences in the degree, all cases showed a form of VO resembling AH with yellow-white brilliant reflective particles. Although the patients complained of floaters, it did not cause significant vision loss, similar to AH. Although the AH and VO that occur after brolucizumab injection are similar in several aspects, brolucizumab induced VO improves while AH does not disappear. The particles in VO produced by brolucizumab are likely to be associated with inflammation, but this is not also certain as they are not accompanied by much inflammation elsewhere. Future histological studies are needed to identify the relationship between the two diseases.
In a previous case report [13], noninfectious vitreous inflammation that occurred after intravitreal bevacizumab injection was treated using 1% prednisolone eye drops and improved within 10 days. In another case [12], noninfectious vitreous inflammation that occurred after intravitreal aflibercept injection was treated with intravitreal antibiotic injection, although the bacterial culture result was negative. In two cases in the present study, the patients received steroid eye drops and oral prednisolone, and VO completely improved after 11 and 14 weeks. However, VO also completely improved after 4 to 8 weeks in the four patients who did not receive treatment. Thus, it seemed that the improvement in VO was not significantly related to any treatment. In two patients with diabetes, it took a relatively long time for VO to improve, at 11 and 14 weeks. To gain a more precise understanding of the association with diabetes, additional studies incorporating a larger number of cases will be necessary. Therefore, if the course of VO is not severe and no other inflammatory findings are observed, monitoring with close follow-up may be sufficient instead of active treatment.
The limitations of this study is that it was conducted at a single institution. Second, the number of patients included in this study was relatively small. Third, because of the short study period, the recurrence rate of VO and the possibility of readministering brolucizumab could not be evaluated. Longitudinal studies with a larger number of cases in the multicenter research will be necessary in the future to obtain more meaningful results.
In conclusion, VO with yellow-white brilliant reflective particles occurred after six of 220 intravitreal brolucizumab injections (2.7%) at our clinic, which reflects a relatively high probability. The VO that occurred after brolucizumab injection at our clinic disappeared after approximately 4 to 14 weeks. Among the six patients, two were treated with oral steroids and topical steroids, but a comparison of the improvement period of VO with patients who did not receive treatment indicated that the treatment did not reduce the duration of VO. Other accompanying inflammation was rarely observed, and VO was not accompanied by other symptoms, including vision impairment, injection, and pain. If a patient complains of floaters after an intravitreal brolucizumab injection, close fundus observation is necessary to evaluate the VO.
Acknowledgements
None
Notes
Conflicts of Interest: None.
Funding: None.