 |
 |
| Korean J Ophthalmol > Volume 38(1); 2024 > Article |
|
Abstract
Purpose
We aimed to compare the accuracy of the intraocular lens (IOL) calculation formula using the standard keratometry (K) and total K (TK) during the femtosecond laser-assisted cataract surgery (FLACS) with a monofocal IOL with enhanced intermediate function using currently used formulas.
Methods
A retrospective review of 125 eyes from 125 patients who had undergone FLACS with implantation of monofocal IOL with enhanced intermediate function was conducted. The predicted refractive power was calculated using an optical biometer (IOLmaster 700) according to the K and TK in the Barrett Universal II, SRK/T, Haigis, and Holladay 2 formulas. Absolute prediction error (APE) obtained from the actual postoperative refractive outcomes and the refractive error predicted in each formula was compared one month after surgery.
Results
Mean APE ranged between 0.29 and 0.39 diopters (D) regardless of the calculation formula and the method of measuring corneal curvature. Significant differences were observed in the APE from the four formulas and the two keratometric measurements (p = 0.014). In a total of 125 eyes from 125 patients, the mean APE was lowest with the Barrett Universal II formula. Across all formulas, both the mean APE and the median APE tended to be lower for K than for TK, although there was no significant difference. Approximately 70% to 80% of the patients were included within 0.5 D of the refractive error across all formulas. The percentage of eyes within 0.5 D of APE outcomes was not statistically different between the K and TK data when using each formula.
The development of cataract surgical techniques and the evolution of the intraocular lens (IOL) power calculation formula have improved the predictability of postoperative refractive outcomes [1]. Obtaining the target refractive outcome by selecting the appropriate IOL can influence the patientŌĆÖs visual quality, the importance of which has been increasingly highlighted over recent years [2].
Most conventional keratometry and topography assume that the cornea is a single surface with a fixed relationship between the anterior and posterior cornea curvature (standard keratometry [K]). However, astigmatism often had to be overcorrected or undercorrected after cataract surgery using this parameter; thus, the need for a new corneal curvature measurement method has emerged [3,4]. The recent development of equipment that independently measures the posterior cornea curvature has allowed for total corneal curvature measurements (total K, TK) encompassing anterior and posterior cornea curvature and corneal thickness. The IOLmaster 700 (Carl Zeiss Meditec AG) is a device that measures TK by combining telecentric three-zone keratometry and swept-source optical coherence tomography (OCT) technology [5]. Therefore, new IOL calculation formulas using TK data have been introduced, and studies investigating its accuracy are underway [6,7].
The desire to improve spectacle-independent far, intermediate, and near vision concurrently after surgery is growing alongside the development of associated technology. Researchers in the field of IOL have made substantial progress over recent decades in developing multifocal IOLs. However, improved visual acuity did not provide sufficient satisfaction in some patients with implanted multifocal IOLs, and dysphotopsia such as halo, glare, and starbursts occurred with impaired quality of vision [8,9]. Monofocal IOL with enhanced intermediate function (Tecnis ICB00, Johnson & Johnson Vision Care Inc) is a new monofocal IOL that could improve the intermediate-distance performance of monofocal IOLs while minimizing the undesired phenomena of multifocal IOLs [10]. This might accelerate neural adaptation and increase the range of patients who would benefit from these IOLs [10]. Monofocal IOL with enhanced intermediate function meets modern needs and expectations with a modified aspheric anterior surface and a continuous power profile [11]. In a recent case series [12], monofocal IOL with enhanced intermediate function was shown to provide satisfactory intermediate-distance spectacle independence while preserving the visual quality of the single-piece monofocal IOL produced by the same manufacturer.
It is uncertain whether using the TK in cataract surgery with monofocal IOL with enhanced intermediate function during femtosecond laser-assisted cataract surgery (FLACS) is more accurate than using the K. Therefore, we aimed to compare the accuracy of the IOL calculation formulas using the K and TK in conventional formulas (Haigis, SRK/T, and Holladay 2) and a new formula developed for TK (Barrett TK Universal II) during the FLACS.
This study was conducted with the approval of the Institutional Review Board of Asan Medical Center, University of Ulsan College of Medicine, which waived the requirement for informed consent (No. 2023-1228). The study adhered to the tenets of the Declaration of Helsinki and followed good clinical practice guidelines.
This study was a retrospective observational case series study involving 125 patients who underwent FLACS (CATALYS Precision Laser System, Johnson & Johnson Vision) with monofocal IOL with enhanced intermediate function at the Department of Ophthalmology at Asan Medical Center (Seoul, Korea). Patients were informed of the characteristics of the existing IOLs before choosing the IOL type and agreeing to implantation of the monofocal IOL with enhanced intermediate function. The inclusion criteria for this study were the acquisition of preoperative biometric and keratometric measurements using the IOLmaster 700, uneventful FLACS, and patients with corrected distance visual acuity (CDVA) of 0.1 logarithm of the minimum angle of resolution (logMAR) or greater at 1 month after surgery. Patients with a history of ocular surgery (excluding cataract surgery of the opposite eye), ocular surface disease, active ocular infection or inflammation, observation of zonular weakness during surgery, and any intraoperative complications such as posterior capsule rupture were excluded. For patients undergoing bilateral cataract surgery, one eye from each patient was randomly selected for inclusion in the analysis.
All patients underwent preoperative workup, including CDVA, manifest refraction, slit-lamp examination of the anterior and posterior segment, specular microscopy, and macular OCT (Spectralis OCT, Heidelberg Engineering GmbH). The K and TK were measured using the IOLmaster 700, and the IOL power calculations were provided. The repeatability of measurements was ensured through the evaluation of all parameters twice by an experienced technician. The K and TK obtained from the IOLmaster 700 were used for IOL power calculation in three conventional formulas (Haigis, SRK/T, and Holladay 2) and a new formula developed for TK (Barrett TK Universal II). Emmetropic IOL power was selected by choosing the predominantly first negative-targeted IOL among all formulas and predicted refractive outcomes were calculated using the K and TK in all formulas.
All patients underwent scheduled cataract surgery, performed by a single skilled surgeon. Before surgery, a topical anesthetic proparacaine eye drop (Paracaine oph soln 0.5%, Hanmi Pharm) was instilled. Cataract extraction was performed through the standard phacoemulsion technique using a phacoemulsifier (WhiteStar Signature Pro, Johnson & Johnson Vision) after the femtosecond laser procedure, including continuous curvilinear capsulorhexis with a diameter of 5.0 mm, crystalline lens fragmentation, and corneal arcuate incisions. Next, monofocal IOL with enhanced intermediate function was implanted into the capsular bag of the eye. The surgery was then concluded after irrigation and aspiration of the anterior chamber to remove the ophthalmic viscoelastic and sutureless hydroclosure of clear corneal incisions with a balanced salt solution. Postoperative medications included topical moxifloxacin 0.5% eye drops (Vigamox, Alcon Laboratories) and prednisolone acetate 0.1% eye drops (Pred Forte, Allergan) four times a day, ketorolac tromethamine 0.45% eye drops (Acuvail, Allergan) two times a day for 3 weeks.
At 1 month after surgery, uncorrected distance visual acuity, manifest refraction, CDVA, topography, and anterior segment OCT were assessed. Absolute prediction error (APE) defines the absolute difference between the actual postoperative refractive outcomes and the refractive error predicted in the IOL calculation formula measured in each formula. The ratio of APE to be included within 0.25, 0.50, 0.75, and 1.00 diopters (D) of each formula was derived from the calculated APE, and the APE from the K and TK for each formula were compared.
Statistical analysis was done using the IBM SPSS ver. 21.0 (IBM Corp). A Wilk-Shapiro test was used to assess the distribution of numerical data. The APEs from the K and TK of all formulas were compared using the Friedman test with Bonferroni post hoc correction for multiple comparisons. Wilcoxon signed-rank tests were used to evaluate the differences between the APEs obtained from the K and TK in each formula. The McNemar test was performed to compare the percentage of eyes within 0.50 D of the APE between the K and TK in each formula. A p-value of <0.05 was considered statistically significant.
Patient demographics and preoperative and postoperative ophthalmologic data are presented in Table 1. The medical records of 125 eyes from 125 patients (mean age, 67.57 ┬▒ 9.65 years; range, 37-83 years) were reviewed. The mean axial length (AXL) was 24.01 ┬▒ 1.47 mm (range, 21.08-28.44 mm), and the mean anterior chamber depth was 3.23 ┬▒ 0.39 mm (range, 2.24-4.04 mm).
There was no significant difference observed between the mean K (44.19 ┬▒ 1.38 D) and TK (44.23 ┬▒ 1.45 D) data measured using the IOLmaster 700 before surgery (p = 0.746). The best-corrected visual acuity improved significantly from 0.43 ┬▒ 0.44 to 0.07 ┬▒ 0.12 logMAR after FLACS with monofocal IOL with enhanced intermediate function (p < 0.001). The preoperative and postoperative spherical equivalents were ŌłÆ1.36 ┬▒ 4.43 and ŌłÆ0.31 ┬▒ 0.43D, respectively (p = 0.032).
Mean APE ranged between 0.29 and 0.39 D regardless of the calculation formula and the method of measuring corneal curvature (K or TK). The Friedman rank test revealed a significant difference when analyzing the APE from the four formulas and the two keratometric measurements (p < 0.001). Post hoc analysis was conducted using the Wilcoxon signed-rank test, and a Bonferroni correction was applied or adjusting multiple comparisons. The resulting significance level was determined to be p < 0.018. The overall comparison and subsequent post hoc analysis revealed that the mean APE was lowest in Barrett Universal II, followed sequentially by the Barrett TK Universal II, Haigis using K, Haigis using TK, SRK/T using K, SRK/T using TK, Holladay 2 using K, and Holladay 2 using TK. When comparing each formula with all others, the APE of both Holladay 2 using K and Holladay 2 using TK were significantly higher than that of all formulas, except for SRK/T using K and SRK/T using TK. Among the other formulas, significant differences were observed between Barrett Universal II and SRK/T using K (adjusted p < 0.001), as well as between Barrett TK Universal II and SRK/T using K (adjusted p < 0.001) (Table 2).
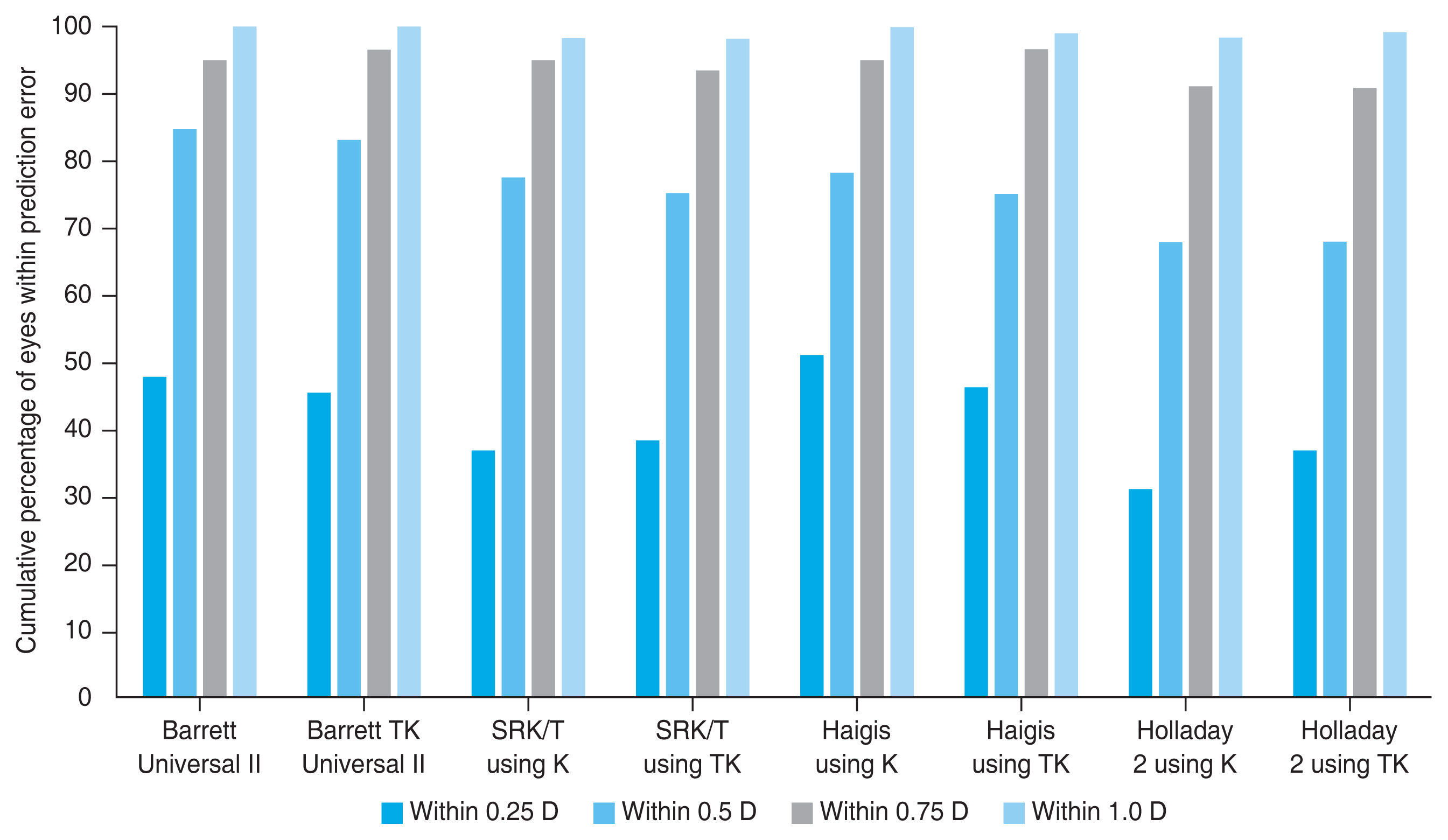
When calculating the ratio of APE to be included within 0.25, 0.50, 0.75, and 1.00 D of each formula, 70% to 80% of patients showed APE within 0.5 D across all formulas. The percentage of eyes within 0.5 D of APE outcomes showed no statistical difference between K and TK data when using each formula ( p = 0.754 for Barrett Universal II, p = 0.508 for SRK/T, p = 0.424 for Haigis, and p > 0.999 for Holladay 2) (Table 2 and Fig. 1).
Table 3 presents the subgroup analysis based on AXL. In the short AXL group (<22.5 mm), the Barrett TK Universal II formula had the lowest mean APE, while the Holladay 2 using K had the highest mean APE. In the long AXL group (>25.5 mm), the Barrett Universal II formula had the lowest mean APE, while the Holliday 2 using K had the highest mean APE.
In the present study, we demonstrated no significant difference in accuracy among IOL calculation formulas when implanting the monofocal IOL with enhanced intermediate function during the FLACS. Additionally, the superiority of the TK data was not revealed compared with the K data, including the Barrett TK Universal II formula. A previous study [6] noted that APE from TK data marginally tended toward higher frequencies in the lower error range than the APE from K data, whereas another study [13] reported that direct measurement of the posterior cornea did not show superior predictability than the prediction of the posterior cornea. One recent study [14] observed that the absolute error using the SRK/T formula was significantly larger than that using the Barrett Universal II formula. They also found a significant correlation between the prediction error and the keratometric readings using the SRK/T formula; however, there was no significant correlation between the prediction error and the keratometric reading using the Barrett Universal II formula. The Barrett Universal II formula has provided higher predictability than the SRK/T formula, especially in eyes with long AXL [15,16]. In our study, there were significant differences among the formulas, and the Holladay 2 formula demonstrated significantly lower refractive predictability.
Mean APE was in ranged from 0.29 to 0.39 D, and approximately 70% to 80% of patients showed APE within 0.5 D regardless of the calculation formula and method of measuring corneal curvature. A large-scale multinational study [17] recently showed that the mean APE after cataract surgery was 0.42 D, and 72% of the study group was included within ┬▒0.50 D of the target spherical equivalent. In our study, there was no statistically significant difference in the percentage of eyes falling within 0.5 D of the APE when comparing K and TK data using each formula.
The monofocal IOL with enhanced intermediate function is a single-piece hydrophobic acrylic IOL designed to smooth and continuously progress its power created by a higher-order asphere from the periphery to the center with no demarcation line [12]. This IOL had an advantage in showing less photic phenomenon and better intermediate visual acuity than monofocal IOL in previous studies [12,18-20]. It also has become a new option between the existing monofocal and multifocal IOLs, and may hold advantages over multifocal IOLs that include less severe photopic phenomena and improved contrast sensitivity [21]. Most formulas currently in use are Gaussian optics-based vergence formulas. These formulas require multiple input variables to calculate IOL power, including effective lens position, corneal power, axial length, vertex distance, and targeted refraction. The number of variables used in each formula vary, from two (Hoffer Q, Holladay 1 and SRK/ T), three (Haigis), five (Barrett Universal II), to up to seven variables (Holladay 2) [22]. According to our results, the existing IOL calculation formula can show excellent predictive accuracy when applied to the monofocal IOL with enhanced intermediate function.
Technologic advancements have allowed for accurate measurement of corneal power, an important variable in IOL calculation. Although anterior and posterior corneal curvature and corneal thickness affect the corneal power, the conventional keratometry and topography measured only anterior corneal curvature [6,23]. Thus, the IOL power was determined through calculations with a predetermined ratio [6,23]. The development of ocular biometers such as the IOLmaster 700 used in this study has allowed for the measurement of total corneal power. Several studies on applying the total keratometry data into IOL power calculation have been conducted [24-27]. One recent study using monofocal IOL [28] reported that the application of TK to a new formula developed for TK increased the accuracy of IOL power calculation. Furthermore, a study on multifocal IOL [26] reported that the accuracy of TK compared with K varied according to the type of IOL and the calculation formula. In our study, the superiority of the TK data did not reveal any superiority over the K data based upon the results that there was no significant difference in the APE when predicting refractive error using the K or TK in each formula.
Previous studies have reported the advantages of applying the TK data in cases with higher astigmatism and post-refractive surgery eyes [3]. In the present study, we observed not only a significant correlation between K and TK measurements but also a tendency toward improved refractive outcomes in the K group compared to the TK group. These results were supported by lower median APE values and a higher percentage of eyes falling within the desired prediction errors, albeit no significant difference. We propose that these unexpected results are more likely attributable to the higher accuracy when using K, rather than a lower accuracy when using TK. In a previous study [26], differences in absolute prediction errors using the K and TK data varied according to the specific type of multifocal IOL. As such, our current findings might also be due to the characteristics of a monofocal IOL with enhanced intermediate function. In addition, recently introduced Barrett Universal II using TK formula was specifically designed for application with TK. However, currently available other IOL calculation formulas relied on the K data from the IOLmaster 700 rather than the TK data. Therefore, using the K data within these formulas would be advantageous over using the TK data. Therefore, it may be essential to gather extensive data on refractive outcomes based on the TK data to advance our understanding and accuracy in IOL power calculation.
This study is limited due to the retrospective character of the study and limited amount of patients, and short follow-up period. Considering that many studies have shown that the surgical equivalent was stabilized 1 to 2 weeks after cataract surgery, we expect that our results of the first month after surgery are unlikely to show a significant difference from the long-term results [29-31]. Nevertheless, long-term follow-up study with many cases is needed to support our conclusion. In this study, we only used the monofocal IOL with enhanced intermediate function in this study, so our results may not be generalizable to the entire range of monofocal IOLs with enhanced intermediate function. Furthermore, the impact of astigmatic changes following femtosecond laser-assisted astigmatic keratotomy may have influenced our data. However, previous studies showed that there is no significant difference in the postoperative refractive outcomes between the FLACS and conventional manual phacoemulsification surgery [32]. To examine the accuracy of the new TK technology, it is required to conduct additional studies with a substantial number of a patients undergoing cataract surgery with FLACS using K and TK.
In conclusion, keratometric measurement considering the posterior corneal curvature (TK) did not show any additional advantages in cataract surgery where the monofocal intraocular lens with enhanced intermediate function was implanted during the FLACS. Additional TK data need to be collected for each formula to enable more precise IOL power calculation during the FLACS with several type IOLs.
Notes
Funding
This work was supported by the Korea Medical Device Development Fund (No. 1711174348, RS-2020-KD000148), granted by the Korean government (the Ministry of Science and ICT; the Ministry of Trade, Industry, and Energy; the Ministry of Health and Welfare; the Ministry of Food and Drug Safety); by the Korean Fund for Regenerative Medicine (No. 21C0723L1-13), funded by the Korean Ministry of Science and ICT and the Korean Ministry of Health and Welfare; by the National Research Foundation of Korea (NRF) grant (No. RS-2023- 00214125), funded by the Korean Ministry of Science and ICT; and by a grant from the Asan Institute for Life Science, Asan Medical Center (No. 2022IP0019-1).
References
1. Song JH, Kang JY, Nam KY, et al. Time series changes in cataract surgery in Korea. Korean J Ophthalmol 2018;32:182-9.




2. Chung JK, Lee HK, Kim MK, et al. Cataract surgery practices in the Republic of Korea: a survey of the Korean Society of Cataract and Refractive Surgery 2018. Korean J Ophthalmol 2019;33:451-7.




3. Savini G, Naeser K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci 2015;56:827-35.


4. Koch DD, Ali SF, Weikert MP, et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg 2012;38:2080-7.


5. Akman A, Asena L, Gungor SG. Evaluation and comparison of the new swept source OCT-based IOLMaster 700 with the IOLMaster 500. Br J Ophthalmol 2016;100:1201-5.



6. Fabian E, Wehner W. Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J Refract Surg 2019;35:362-8.


7. Kern C, El Kaissi L, Kortuem K, et al. Comparing refractive outcomes of a standard industry toric IOL calculator using anterior corneal astigmatism and total corneal refractive power. Graefes Arch Clin Exp Ophthalmol 2020;258:345-50.



10. Mencucci R, Favuzza E, Caporossi O, et al. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol 2018;256:1913-22.



11. Tognetto D, Cecchini P, Giglio R, Turco G. Surface profiles of new-generation IOLs with improved intermediate vision. J Cataract Refract Surg 2020;46:902-6.


12. Mencucci R, Cennamo M, Venturi D, et al. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: preliminary results. J Cataract Refract Surg 2020;46:378-87.


13. Ferreira TB, Ribeiro P, Ribeiro FJ, OŌĆÖNeill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg 2017;33:794-800.


14. Iijima K, Kamiya K, Iida Y, Shoji N. Comparison of predictability using Barrett universal II and SRK/T formulas according to keratometry. J Ophthalmol 2020;2020:7625725.




15. Abulafia A, Barrett GD, Rotenberg M, et al. Intraocular lens power calculation for eyes with an axial length greater than 26.0 mm: comparison of formulas and methods. J Cataract Refract Surg 2015;41:548-56.


16. Zhang Y, Liang XY, Liu S, et al. Accuracy of intraocular lens power calculation formulas for highly myopic eyes. J Ophthalmol 2016;2016:1917268.




17. Lundstrom M, Dickman M, Henry Y, et al. Risk factors for refractive error after cataract surgery: analysis of 282 811 cataract extractions reported to the European Registry of Quality Outcomes for cataract and refractive surgery. J Cataract Refract Surg 2018;44:447-52.


18. Unsal U, Sabur H. Comparison of new monofocal innovative and standard monofocal intraocular lens after phacoemulsification. Int Ophthalmol 2021;41:273-82.



19. Kang KH, Song MY, Kim KY, et al. Visual performance and optical quality after implantation of a new generation monofocal intraocular lens. Korean J Ophthalmol 2021;35:112-9.




20. Auffarth GU, Gerl M, Tsai L, et al. Clinical evaluation of a new monofocal IOL with enhanced intermediate function in patients with cataract. J Cataract Refract Surg 2021;47:184-91.


21. Cochener B; Concerto Study Group. Clinical outcomes of a new extended range of vision intraocular lens: International Multicenter Concerto Study. J Cataract Refract Surg 2016;42:1268-75.


22. Kane JX, Chang DF. Intraocular lens power formulas, biometry, and intraoperative aberrometry: a review. Ophthalmology 2021;128:e94-114.


23. Olsen T. On the calculation of power from curvature of the cornea. Br J Ophthalmol 1986;70:152-4.



24. LaHood BR, Goggin M. Measurement of posterior corneal astigmatism by the IOLMaster 700. J Refract Surg 2018;34:331-6.


25. Chung HS, Chung JL, Kim YJ, et al. Comparing prediction accuracy between total keratometry and conventional keratometry in cataract surgery with refractive multifocal intraocular lens implantation. Sci Rep 2021;11:19234.




26. Lee H, Chung JL, Kim YJ, et al. Prediction accuracy of standard and total keratometry by swept-source optical biometer for multifocal intraocular lens power calculation. Sci Rep 2021;11:4794.




27. Ryu S, Jun I, Kim TI, et al. Prediction accuracy of conventional and total keratometry for intraocular lens power calculation in femtosecond laser-assisted cataract surgery. Sci Rep 2021;11:12869.




28. Srivannaboon S, Chirapapaisan C. Comparison of refractive outcomes using conventional keratometry or total keratometry for IOL power calculation in cataract surgery. Graefes Arch Clin Exp Ophthalmol 2019;257:2677-82.



29. Charlesworth E, Alderson AJ, de Juan V, Elliott DB. When is refraction stable following routine cataract surgery? A systematic review and meta-analysis. Ophthalmic Physiol Opt 2020;40:531-9.



30. Ostri C, Holfort SK, Fich MS, Riise P. Automated refraction is stable 1 week after uncomplicated cataract surgery. Acta Ophthalmol 2018;96:149-53.



Fig.┬Ā1
The cumulative percentage of eyes within the specific range of predicted postoperative spherical equivalent refraction outcomes for the different formulas, including the Barrett Universal II, Barrett TK Universal II, SRK/T, Haigis, and Hollday 2 formulas. D, diopters.
Table┬Ā1
Demographics and ocular biometric data of enrolled patients
Table┬Ā2
Absolute prediction error between standard K and TK using Barrett Universal II, Barrett TK Universal II, SRK/T, Haigis, and Holladay 2 formulas (n = 125)
Table┬Ā3
Mean absolute prediction error in each axial length subgroup according to the formulas



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




