 |
 |
| Korean J Ophthalmol > Volume 37(6); 2023 > Article |
|
Abstract
Purpose
Methotrexate (MTX) is an immunosuppressive agent used to treat noninfectious inflammatory eye conditions and is generally administered orally for ocular inflammatory diseases. When used in rheumatological diseases, subcutaneous administration has been reported to show higher efficacy than oral administration. Therefore, this study aimed to evaluate the effect of subcutaneous MTX in patients with refractory uveitis or choroiditis who did not respond to other immunosuppressive agents.
Methods
A retrospective case series study was performed between January and December 2018. Patients with uveitis or chorioretinitis who showed little to no treatment response for 6 months or more with conventional immunosuppressive agents were treated with MTX, administered subcutaneously. After 6 months of treatment, patients were evaluated to determine whether complete suppression of inflammation sustained for ≥28 days was achieved in both eyes and whether improvement can be confirmed by fluorescein angiography (FAG).
Results
Subcutaneous MTX treatment was performed on 18 patients: 11 had intermediate uveitis and seven had posterior uveitis. In the intermediate uveitis patient group, five patients (50% of the group excluding one patient who dropped out) showed improvement in FAG and three patients (30%) showed complete suppression of inflammation. In the posterior uveitis group, two out of seven patients (excluding two patients who dropped out) showed an improvement, two patients in the group showed little change, and one patient showed aggravation of FAG findings.
In general, for noninfectious inflammatory eye conditions, ophthalmologists consider the use of corticosteroids as the first choice [1]. However, if corticosteroids do not show effective control of inflammation, or if inflammation persists and long-term use of corticosteroids is required, immunosuppressive agents are used to avoiding the risk of adverse effects from the use of corticosteroids [2]. Immunosuppressive agents are classified as antimetabolites, T-cell inhibitors, alkylating agents, and biologic agents (anti-tumor necrosis factor agents). The representative antimetabolites include methotrexate (MTX), mycophenolate mofetil, and azathioprine; T-cell inhibitors include cyclosporin and tacrolimus; alkylating agents include cyclophosphamide; and biologic agents include adalimumab and infliximab [3,4].
Treatment strategies for uveitis have diversified options due to the development and introduction of various immunosuppressants, but still have many problems. Many patients do not respond to treatment or relapse, and visual impairment accumulates as uveitis attacks are repeated. Refractory uveitis has a high risk of vision loss due to persistent inflammation or repeated recurrence despite the combined use of immunosuppressive drugs, and side effects due to long-term use of immunosuppressive drugs are often accompanied. Therefore, more effective and safe treatment methods are required.
MTX is one of the oldest and most commonly used immunosuppressive agents for noninfectious inflammatory eye diseases [1]. MTX inhibits dihydrofolate reductase and interferes with its conversion to tetrahydrofolate which is necessary for the synthesis of purine and pyrimidine; it exhibits an antiproliferative effect, inhibits aminoimidazole carboxamide ribonucleotide transformylase, and releases adenosine that results in an anti-inflammatory effect. Typically, a dose-escalation regime (that starts at 7.5-12.5 mg/week and escalates to 25 mg/week) is used, and folic acid is taken on days when MTX is not administered to reduce the adverse effects of tetrahydrofolate depletion [2].
MTX is usually administered orally, but it is also administered subcutaneously, intravenously, or intravitreally [5]. There is a limited number of reports on subcutaneous MTX to treat ophthalmic conditions but research in rheumatic medicine has reported that subcutaneous MTX has higher efficacy and bioavailability and less gastrointestinal distress than oral administration; patients taking oral MTX have reported better effects after switching to subcutaneous injection [6].
The present study aimed to determine the effect of subcutaneous MTX in patients with refractory uveitis. We investigated whether subcutaneous administration of MTX could be an alternative for patients who did not respond to other immunosuppressant treatments, including oral methotrexate, or who could not continue treatment due to side effects.
This study was approved by the Institutional Review Board of Nune Eye Hospital (No. 1812-001-999). The study complied with the tenets of the Declaration of Helsinki. All participants provided written informed consent.
We performed a retrospective analysis on patients who underwent subcutaneous MTX treatment between January and December 2018 in Nune Eye Hospital (Seoul, Korea). Subcutaneous MTX treatment was performed in patients with intermediate or posterior uveitis that showed little or no response to conventional oral immunosuppressive agents, but excluding biologic agents such as adalimumab, for more than 6 months. Patients with glaucoma, proliferative or severe nonproliferative diabetic retinopathy, and other diseases that can cause macular damage or optic nerve damage such as neovascular age-related macular degeneration were excluded from the study.
Before starting the first subcutaneous MTX administration, the interferon-γ release assay was performed to detect latent tuberculosis infection and complete blood count (CBC) and chemistry profile were performed to screen for hematologic disorders. The CBC and chemistry profile were performed monthly for up to 3 months and then between 2 and 4 months, depending on the patient’s condition, for continuous monitoring of hematologic disorders. The subcutaneous dose was 20 mg MTX/wk; it was self-injected using a 31-gauge insulin syringe. Patients were trained to self-inject around the lower abdomen or thighs once a week. Folic acid (1 mg daily) was prescribed, excluding the days of injection, to reduce the adverse effects of MTX and also recommended to consume caffeine (coffee or dark chocolate) to reduce the symptoms of MTX intolerance [7].
Patients were evaluated 6 months after treatment to determine whether complete suppression of inflammation sustained for ≥28 days in both eyes was attained and improvement on fluorescein angiography (FAG) was visible. The complete suppression of inflammation was defined using the Standardization of Uveitis Nomenclature Working Group criteria with anterior chamber cell grade ≤0.5, and posterior inflammation and activity of posterior uveitis was assessed according to the FAG scoring system published by the Angiography Scoring for Uveitis Working Group [8].
In evaluating FAG, “improved” was defined as FAG scoring of both eyes improved by 4 points or more compared to baseline or FAG scores at 6 months was decreased and less than 4 points; “aggravated” was defined when FAG scoring deteriorated by 4 points or more compared to baseline in either eye; and when the score change was less than 4 points, the patient condition was defined as “stable.”
In cases where the corticosteroids dose could not be reduced to 5 mg or less up to 6 months or 3 months after the use of subcutaneous MTX, other treatments such as subtenon triamcinolone, intravitreal triamcinolone, and adalimumab were added due to worsening of inflammation; the patient condition was defined as “aggravated.”
During the study period, a total of 18 patients underwent subcutaneous MTX treatment; 11 with intermediate uveitis and seven with posterior uveitis. The mean age of the patients was 44.3 years; there were nine men and nine women.
As a result of monitoring patients with intermediate uveitis for 6 months, five out of 10 patients (50%), except for the one who dropped out after 1 month, showed improvement in FAG findings, and three patients (30%) showed complete suppression of inflammation (Table 1).
In patients with posterior uveitis, two out of five patients (excluding two patients who dropped out) showed an improvement, two patients showed little change, and one patient showed aggravation of FAG findings (Table 2).
The FAG and fundus photographs of case 1 showed the leakage of the disc, macular, arcade, and capillary observed in the initial FAG improved after 6 months (Fig. 1A-1H). It can be seen that the disc leakage observed in posterior uveitis patient (case 12) also improved after 6 months (Fig. 2A-2C).
Of the 18 patients, complications occurred in five patients. Four patients developed urticaria and edema considered to be allergic reactions: two of the patients with severe edema of low extreme were excluded from the study and the other two showed improvements after the transient occurrence of urticaria and continued with the subcutaneous MTX treatment. Gastrointestinal problems developed in one patient. Three patients dropped out without completing 6 months of treatment. Other serious adverse events were not observed.
In previous studies, oral administration of MTX was less effective than mycophenolate and azathioprine and resulted in higher discontinuation rates due to side effects [9]. However, subcutaneous MTX has been reported to have higher efficacy than oral MTX in the treatment of rheumatoid arthritis, presumably due to the higher bioavailability of MTX given subcutaneously [6,10]. According to a previous study by Braun et al. [6], changing to subcutaneous MTX in patients with rheumatoid arthritis who did not achieve the treatment target with oral MTX resulted in 30% of the patients achieving the treatment target.
The difference in efficacy according to the route of administration of MTX is due to its bioavailability. At a low dose (<15 mg/m2/wk), oral administration showed 11% to 15% lower bioavailability on average compared to the subcutaneous route, and showed a highly variable tendency, especially with large individual variation [11]. At high dose (≥15 mg/m2/wk), this difference widens further, and oral administration reduces the effect by about one-third [12,13]. This is due to the low and saturating problem of intestinal absorption of MTX, limiting the bioavailability of oral administration. Oral administration no longer increases plasma methotrexate concentration at doses above 15 mg/wk.
In this study, improvement of refractory uveitis was observed by changing the route of administration of MTX from oral to subcutaneous in two children. Although the dose was increased by 2.5 to 5.0 mg, stable increase in bioavailability can be expected when changing to subcutaneous administration in case of poor intestinal absorption. In refractory uveitis in which inflammation is not controlled, changing the route of administration of MTX can relieve inflammation and bring about remission. It is also easy to further increase the administration dose.
Oral MTX causes side effects such as gastrointestinal distress, nausea, and elevated liver enzyme. Although not a serious complication, it makes difficult to continue treatment. Despite being effective, 34% of rheumatoid arthritis patients discontinued MTX treatment within 2 years due to side effects [5]. Among them, gastrointestinal symptoms were identified with a high frequency of 30.8%. Subcutaneous MTX does not pass through the intestines, causes fewer gastrointestinal side effects [11]. In addition, nausea could be reduced by caffeine intake, so the continuity of MTX treatment could be increased.
In this study, out of 18 patients with uveitis or chorioretinitis who had a refractory response to other oral immunosuppressive agent treatment, 46.6% of patients (excluding three dropouts) showed improvement in FAG findings, and out of 11 patients with intermediate uveitis, 30% (excluding one dropout) showed complete suppression of inflammation. The findings of this study can be considered to have clinical significance in that the effect of changing to subcutaneous MTX was demonstrated in patients who showed refractory treatment responses to other immunosuppressive agents.
Newly developed biologic agents such as adalimumab are being used to treat refractory uveitis with some success [14,15]. However, concomitant use of MTX is required to suppress the anti-adalimumab antibody that causes drug resistance [16]. MTX is also the most commonly used medication in children, with proven safety and effectiveness [5,17]. Safety is a key issue when considering changes to other immunosuppressive agents, especially in the treatment of pediatric patients with chronic uveitis. Before considering the change in the use of new biologic agents, a change to subcutaneous MTX is thought to be a reasonable option.
The results of this study confirmed that subcutaneous MTX in uveitis or chorioretinitis patient refractory to treatment with other immunosuppressive agents was effective to treat these conditions. By changing the route of MTX administration, it is possible to obtain a more effective and stable increase in plasma methotrexate concentration while reducing side effects, providing an alternative treatment for refractory uveitis.
This study has limitations in that it is a simple case series, and there was no comparison of the effect of subcutaneous MTX with a control group; thus, the findings should be interpreted with care. Further prospective, randomized, large-scale, and long-term follow-up studies are needed.
References
1. Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology 2009;116:2188-98.



2. Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000;130:492-513.


3. Barry RJ, Nguyen QD, Lee RW, et al. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol 2014;8:1891-911.


4. Gomez-Gomez A, Loza E, Rosario MP, et al. Efficacy and safety of immunomodulatory drugs in patients with non-infectious intermediate and posterior uveitis, panuveitis and macular edema: a systematic literature review. Semin Arthritis Rheum 2020;50:1299-306.


5. Woo SJ, Kang EH. Use of methotrexate for the treatment of ocular inflammation and uveitis. J Pharmacovigil 2013;1:117.
6. Braun J, Kastner P, Flaxenberg P, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis Rheum 2008;58:73-81.

7. Malaviya AN. Methotrexate intolerance in the treatment of rheumatoid arthritis (RA): effect of adding caffeine to the management regimen. Clin Rheumatol 2017;36:279-85.



8. Tugal-Tutkun I, Herbort CP, Khairallah M; Angiography Scoring for Uveitis Working Group (ASUWOG). Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis). Int Ophthalmol 2010;30:539-52.



10. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol 2004;31:645-8.

11. Bianchi G, Caporali R, Todoerti M, Mattana P. Methotrexate and rheumatoid arthritis: current evidence regarding subcutaneous versus oral routes of administration. Adv Ther 2016;33:369-78.



12. Tukova J, Chladek J, Nemcova D, et al. Methotrexate bioavailability after oral and subcutaneous dministration in children with juvenile idiopathic arthritis. Clin Exp Rheumatol 2009;27:1047-53.

13. Balis FM, Mirro J Jr, Reaman GH, et al. Pharmacokinetics of subcutaneous methotrexate. J Clin Oncol 1988;6:1882-6.


14. Suhler EB, Jaffe GJ, Fortin E, et al. Long-term safety and efficacy of adalimumab in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology 2021;128:899-909.


15. Suhler EB, Adan A, Brezin AP, et al. Safety and efficacy of adalimumab in patients with noninfectious uveitis in an ongoing open-label study: VISUAL III. Ophthalmology 2018;125:1075-87.


Fig. 1
Case 1 images. (A,B) Fundus photo at baseline. (C,D) Fluorescein angiography image at baseline. (E,F) Fundus photo after 6 months of treatment with subcutaneous methotrexate. (G,H) Fluorescein angiography image after 6 months of treatment with subcutaneous methotrexate.
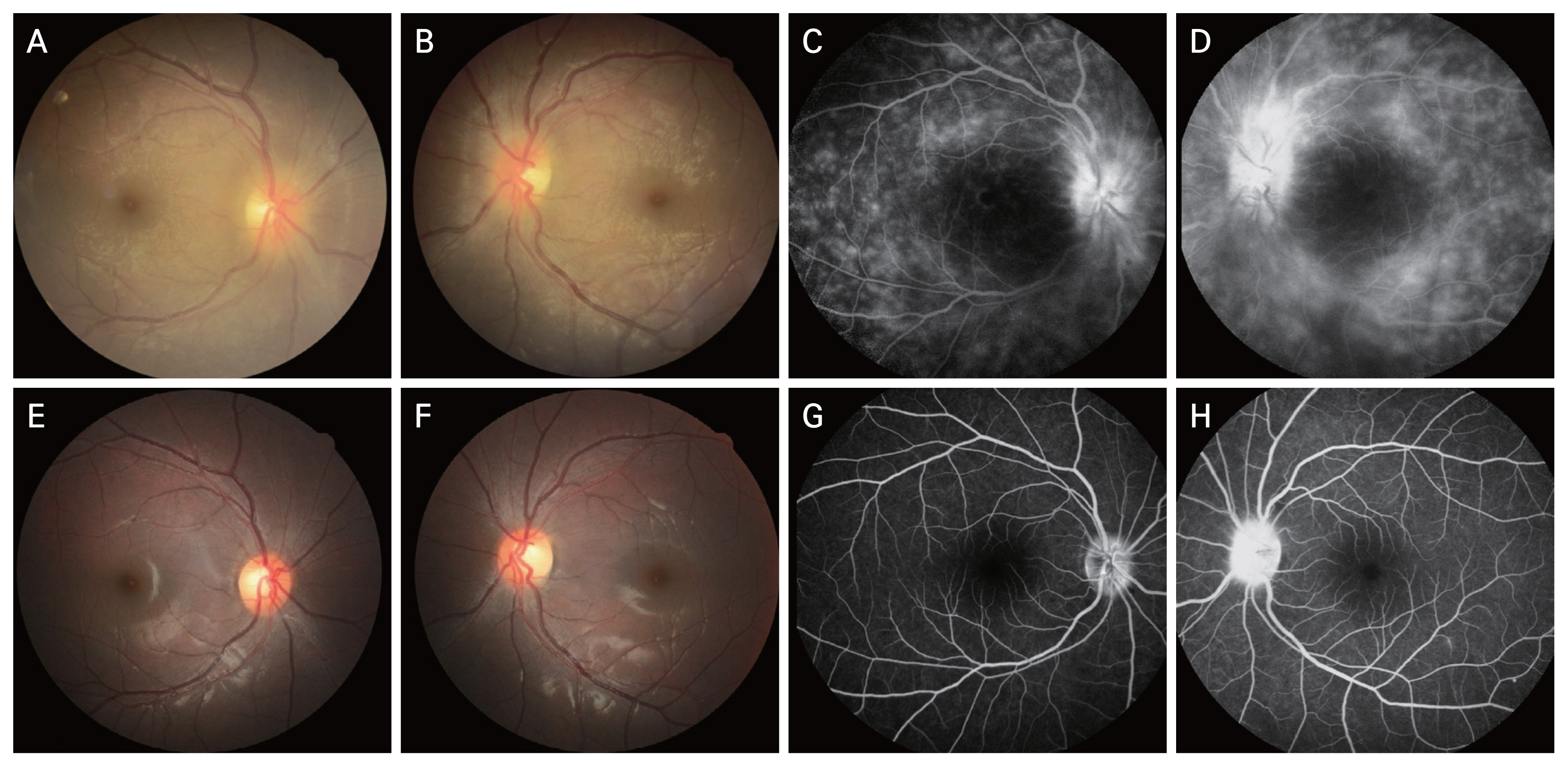
Fig. 2
Case 12 fluorescein angiography images at (A) baseline, (B) 1 month, and (C) 6 months of subcutaneous methotrexate.
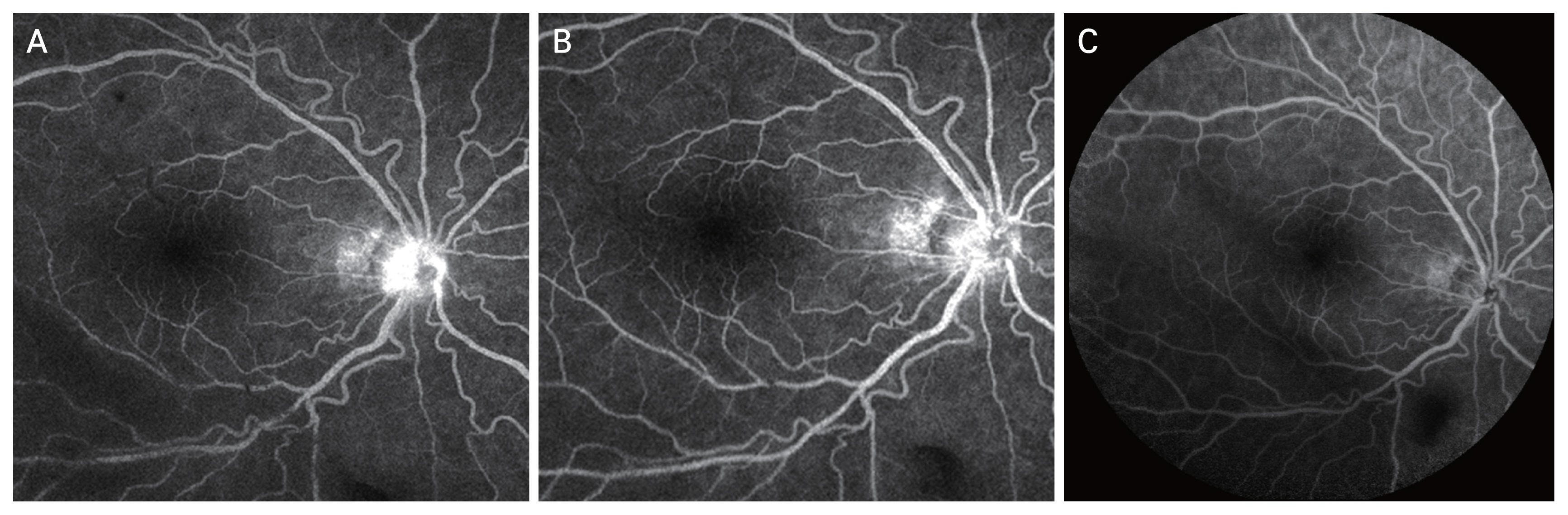
Table 1
Intermediate uveitis patients treated by subcutaneous MTX
Table 2
Posterior uveitis patients treated by subcutaneous MTX



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




