 |
 |
| Korean J Ophthalmol > Volume 37(5); 2023 > Article |
|
Abstract
Purpose
To evaluate the usefulness of a newly generated monofocal intraocular lens (IOL) in patients with various retinal diseases who underwent combined cataract and pars plana vitrectomy surgery.
Methods
This prospective observational study included 33 patients with various retinal diseases. Monocular best-corrected distance visual acuity (BCDVA), uncorrected distance visual acuity (UCDVA), uncorrected intermediate visual acuity (UCIVA), uncorrected near visual acuity (UCNVA), and contrast sensitivity were measured and compared with 40 age-matched patients in the standard monofocal IOL.
Results
The Eyhance IOL group demonstrated significantly better UCIVA at 6 months follow-up compared to the standard monofocal IOL group. No significant differences were observed between the two groups in contrast sensitivity, BCDVA, UCDVA, or UCNVA. The regression analysis showed a significant association between preoperative corrected distance visual acuity and improved UCIVA in the Eyhance IOL group.
Cataract extraction with intraocular lens (IOL) implantation is a surgery that has been commonly performed and tremendously developed in the field of ophthalmology. Techniques for cataract surgery as well as the quality of IOLs are steadily improving along with increasing patientsŌĆÖ expectations. These expectations include having a good vision without spectacles at all distances (near, intermediate, and far), which leads to the development of multifocal IOLs. Although various types of multifocal IOLs have been developed and preserved visual acuity of patients for more than two types of aforementioned distances, there is a drawback of multifocal IOLs that potentially compromise contrast sensitivity and induce glare and halo [1,2]. These disadvantages become more prominent in patients with vitreoretinal disease requiring surgery, so implanting multifocal IOLs is not recommended for patients with vitreoretinal disease. Thus, the majority of IOLs currently being implanted in combined surgery with cataract and pars plana vitrectomy (PPV) are still monofocal IOLs [3-5].
The Tecnis Eyhance ICB00 IOL (Johnson & Johnson Vision) is a recently developed monofocal IOL aimed at distinguishing design with continuous change in refractory power from the periphery to the center of the IOL [6]. This feature creates a small central zone in the anterior surface of the IOL, extending the depth of focus and consequently maintaining intermediate vision without lowering the quality of vision at distance. Previous comparative studies between Eyhance IOL and classic monofocal IOL revealed that this new monofocal IOL showed better intermediate vision than the standard IOL with similar performance and dysphotopsia profile at far vision [7-11]. In spite of these encouraging characteristics, there is a limited number of studies that have assessed the clinical outcomes of utilizing the Eyhance IOL in patients with retinal diseases.
We designed this prospective study to investigate whether the new innovative monofocal IOL (Eyhance IOL) can improve vision and contrast sensitivity in patients with retinal diseases. We conducted an evaluation of early visual outcomes following the surgery, focusing on uncorrected far, intermediate, and near vision, as well as refraction and contrast sensitivity.
This prospective, nonrandomized, comparative study was conducted in the Department of Ophthalmology at Hallym University Dongtan Sacred Heart Hospital between August 2021 and March 2022, in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Institutional Review Board of Hallym University Dongtan Sacred Heart Hospital (No. HDT 2021-05-004). Informed consent was obtained from all the patients after receiving full disclosure regarding the study.
This prospective study included a total of 73 eyes from patients who underwent uncomplicated cataract and vitreoretinal surgery. The patients were divided into two groups depending on the surgical procedure: Eyhance ICB00 group (implemented Tecnis Eyhance ICB00) and Tecnis ZCB00 group (implemented standard monofocal IOL, Tecnis ZCB00, Johnson & Johnson Vision). Inclusion criteria for the study were the following: age >40 years, impaired visual acuity due to cataract, surgically indicated vitreoretinal disease, and a preoperative corneal astigmatism of 1.0 diopters or less. Exclusion criteria were as follows: history of trauma or ocular surgery, corneal irregularity or abnormality, subluxated and dislocated crystalline lens, presence of uveitis, and intraocular pressure >21 mmHg. Furthermore, patients with intraoperative complications, such as anterior capsule radial tear, zonular dialysis, and posterior capsule rupture, or with postoperative complications, including IOL dislocation, corneal endothelial decompensation, vitreous hemorrhage (VH), detachment of the retina and/or choroid, cystoid macular edema, and recurrence of epiretinal membrane (ERM), were excluded.
All operations were performed by a single surgeon (IHH) under retrobulbar or general anesthesia. A 23-gauge three-port PPV and clear corneal incision phacoemulsification with the Eva Vitrectomy System (DORC Inc) was performed in all patients. After anesthesia, 23-gauge valved trocars were inserted in the inferotemporal (infusion line), superonasal, and superotemporal quadrant 3.5 mm posterior to the limbus. Following routine phacoemulsification surgery with a 2.8-mm clear corneal incision in the superior quadrant, the Eyhance IOL was implanted in the bag. Corneal incisions were sealed by hydration without sutures. This was followed by sequential vitreoretinal surgery. Various techniques depending on vitreoretinal pathology were used at the surgeonŌĆÖs discretion, including core and peripheral vitrectomy, vitreous shaving, epiretinal and/or internal limiting membrane (ILM) peeling, photocoagulation, endodiathermy, using perfluorocarbon liquids, fluid-air exchange, and silicone oil or gas insertion. The trocars were removed by the end of procedures and the sclerotomies were sutured with 8-0 vicryl if necessary. The tightness of all incision sites was checked. A subconjunctival injection of dexamethasone was administered. In the postoperative period, topical antibiotics, corticosteroids, and nonsteroidal anti-inflammatory drugs were administered to all patients four times a day for 1 month [12], and ointment containing neomycin sulfate, polymyxin B sulfate, and dexamethasone (Forus ophthalmic ointment, Samil) was administered at bedtime for 7 days.
Preoperatively, all patients underwent a comprehensive ophthalmic examination, including measurement of uncorrected and corrected visual acuity at 4 m distance, slit-lamp biomicroscopy, Goldmann applanation tonometry, fundus examination, manifest refraction, optical biometry, corneal topography, and macular optical coherence tomography (OCT; Spectralis, Heidelberg Engineering). The power of the IOL to be implanted was based on biometry data measured by IOLMaster 500 (Carl Zeiss Meditec). If the biometry data were not measured by the IOL Master (e.g., VH), contact A-scan biometry were used. Patients for whom biometry data were not obtained, were excluded from the study. IOL power was calculated to target emmetropia for all eyes in the study using the Barret Universal II formula.
Routine postoperative examinations were performed at 1 day, 1 week, and 1, 3, and 6 months after surgery. The results at the 6-month follow-up visit were reported in which manifest refraction, monocular best-corrected distance visual acuity (BCDVA), uncorrected distance visual acuity (UCDVA), uncorrected intermediate visual acuity (UCI-VA), and uncorrected near visual acuity (UCNVA) were noted. Distance visual acuity was measured at 4 m using Early Treatment Diabetic Retinopathy Study (ETDRS) charts (Precision Vision). Intermediate and near visual acuity were measured using the Sloan ETDRS Format Near Vision Chart (Precision Vision) at 66 and 40 cm, respectively. All visual acuities were measured under photopic conditions with 100% contrast. The measured values of visual acuity were converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analyses.
The contrast sensitivity was recorded using a Metrovision MonPack Vision Monitor (P├®renchies). Monocular, corrected distance contrast sensitivity test was performed at postoperative 6 months under both photopic and mesopic conditions. The contrast sensitivity was measured using vertical sinusoidal bars at various spatial frequencies. Each bar was first presented at a low contrast, and then the contrast was progressively increased by the instrument. The point at which the patient first recognized the grating bars was recorded. The Metrovision contrast sensitivity test was carried out at 0.6, 1.1, 2.2, 3.4, 7.1, and 14.2 cycles per degree spatial frequencies and at lumination levels of 0 to 30 dB.
The values of BCDVA and OCT before and 6 months after surgery were compared using a paired t-test. We recruited 30 eyes from 40 age-matched individuals whose UCDVA was more than 0 logMAR as controls. Uncorrected distance, intermediate, and near visual acuities of the patients after surgery were compared with those of the Tecnis ZCB00 group using the Mann-Whitney U-test. The value of the contrast sensitivity test was also compared between the Eyhance ICB00 and Tecnis ZCB00 groups. A regression analysis was performed to identify the factors that may affect the postoperative intermediate visual acuity. All statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Corp). Statistical significance was set at p < 0.05.
The 33 patients in the study group had a mean age of 59.42 ┬▒ 8.55 years (range, 47-71 years) and 21 patients were women and 12 patients were men. Forty subjects (26 women, 14 men) were recruited for the Tecnis ZCB00 group and had a mean age of 62.40 ┬▒ 13.39 years. There was no difference in age (p = 0.953) and sex distribution (p = 0.904) between the two groups (Table 1).
The major concomitant vitreoretinal diseases requiring vitrectomy were VH (24 patients), ERM (17 patients), rhegmatogenous retinal detachment (RRD; 13 patients), vitreomacular traction (two patients), macular hole (five patients), vitreous opacity (12 patients). The reasons for VH were diabetic retinopathy (14 patients), retinal vein occlusion (three patients), retinal tear (one patient), and wet age-related macular degeneration (six patients). The disease distribution did not vary between the Eyhance ICB00 and the Tecnis ZCB00 groups (p = 0.511) In cases of RRD and macular hole, 18% sulfur hexafluoride was used as a tamponade. There was no patient using silicone oil as a tamponade. The average surgery time was 49.39 ┬▒ 9.13 minutes in the Eyhace ICB00 group and 48.53 ┬▒ 20.66 minutes in the Tecnis ZCB00 group. Complications associated with IOL were not observed in both groups.
The average of preoperative BCDVA in the Eyhance ICB00 group was 0.94 ┬▒ 0.93 logMAR and postoperative BCDVA was 0.13 ┬▒ 0.14 logMAR. There was a significant improvement in BCDVA at 6 months follow-up (p < 0.001). The mean preoperative central macular thickness (CMT) was 402.10 ┬▒ 167.79 ╬╝m. Macular OCT was not detected in 12 patients, because of nine patients with VH and three patients with macula-off RRD. The mean CMT after 6 months of operation was 301.33 ┬▒ 80.56 ╬╝m and changes were statistically significant (p = 0.0004). The average of axial length was 23.91 ┬▒ 1.28 mm and the average of postoperative spherical equivalent (SE) was ŌłÆ0.35 ┬▒ 0.49. There was no significant relationship between groups in terms of pre- and post-SE and preoperative BCDVA, whereas the CMT value at 6 months after surgery was significantly lower in the Eyhance ICB00 group (p = 0.017) (Tables 1, 2)
The mean UCDVA, UCIVA, and UCNVA of the Eyhance ICB00 group after surgery were 0.19 ┬▒ 0.14, 0.29 ┬▒ 0.14, and 0.54 ┬▒ 0.20 logMAR, respectively; the mean postoperative UCDVA, UCIVA, and UCNVA of the Tecnis ICB00 group were 0.25 ┬▒ 0.27, 0.49 ┬▒ 0.28, and 0.62 ┬▒ 0.28 logMAR, respectively (Table 2). The UCIVA of the Eyhance ICB00 group was significantly better than those of the Tecnis ZCB00 group (p = 0.001). The postoperative UCIVA:UCDVA ratio was 0.81 ┬▒ 0.11 in the Eyhance ICB00 group and 0.66 ┬▒ 0.20 in the Tecnis ZCB00 group, which was significantly different (p < 0.001).
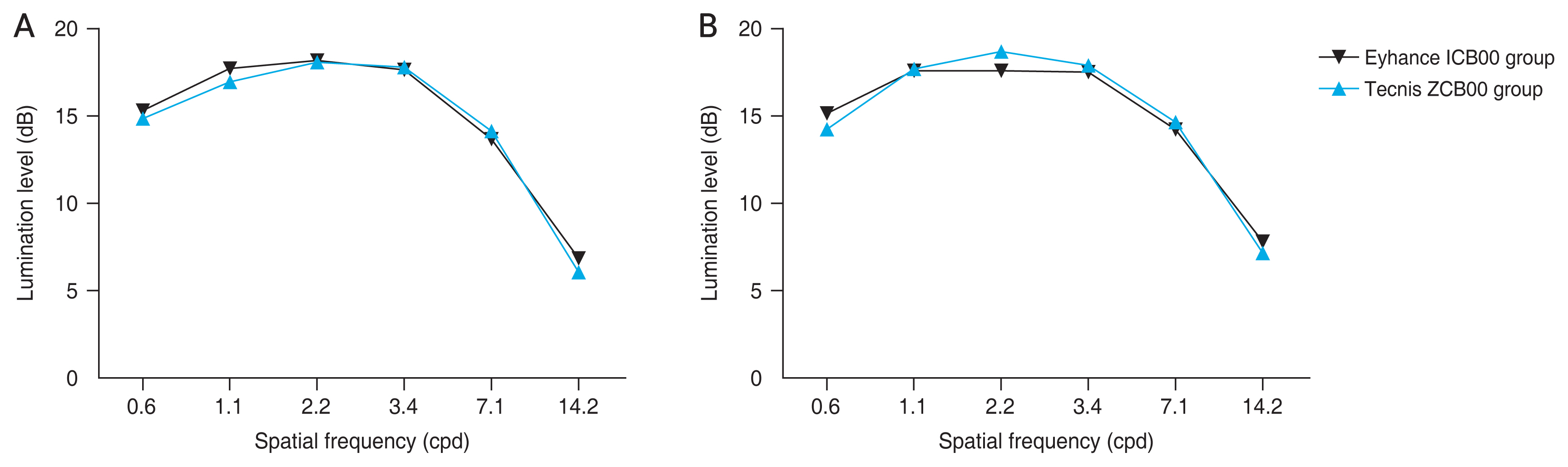
Fig. 1A and 1B displays the average mesopic and photopic contrast sensitivity values for the Eyhance ICB00 group and the Tecnis ZCB00 group. The statistical analysis demonstrated that there were no significant differences between the two groups in terms of mesopic and photopic contrast sensitivity for any spatial frequency. Table 3 contains the results of the regression analysis for the postoperative visual outcomes. Regarding postoperative UCIVA, it was found that preoperative BCDVA had a significant association with the Eyhance ICB00 group.
While the Eyhance IOL is designed as a monofocal IOL, due to the unique continuous refectory change by higher order spheres, it can enhance intermediate vision after cataract surgery [6,13]. Previous studies comparing Eyhance IOL with standard monofocal IOL demonstrated the superior performance of Eyhance IOL, providing significant improvement of intermediate vision without compromising distance and near visual acuity and photic phenomena [7-11]. Eyhance IOL has been considered as an advanced monofocal IOL that provides additional advantages of enhanced intermediate vision while maintaining the benefits of monofocal IOL. There is limited research on the utilization of the Eyhance IOL, not only in simultaneous surgeries with cataract vitrectomy but also in patients with retinal disorders. Our study showed that combined cataract surgery and vitrectomy with Eyhance IOL in patients with retinal disorders effectively improved far vision without compromising contrast sensitivity and also promised intermediate vision.
The study compared the outcomes of patients who underwent combined cataract surgery and vitrectomy and received either the Eyhance ICB00 IOL or the Tecnis ZCB00 IOL. The results indicated that there were no significant differences between the two groups in terms of contrast sensitivity, SE, BCDVA, UCDVA, or UCNVA, but CMT and UCIVA were significantly better in the Eyhance ICB00 group. The Eyhance ICB00 group showed significantly lower CMT, and seemed to exhibit better visual acuity compared to the Tecnis ZCB00 group. Due to the possibility that the better intermediate vision in the Eyhance ICB00 group might be influenced by the better distance vision after surgery, the UCDVA:UCIVA ratio was calculated to eliminate the influence of better UCDVA. The results still indicated higher values in the Eyhance ICB00 group, suggesting that the better UCIVA in this group was not solely attributed to the better UCDVA. The regression analysis conducted on patients using the Eyhance IOL revealed a significant association between better preoperative BCDVA and improved UCIVA at the postoperative follow-up. The findings imply that retinal patients with superior preoperative visual acuity may experience enhanced intermediate vision by opting for the Eyhance IOL over a monofocal IOL.
One of the major reasons of hesitation for the use of multifocal IOL in cataract surgery performed together with vitrectomy is that difficult situations can be encountered during surgery through multifocal IOL. The multiple concentric optical zones with different refractive powers of these IOLs interfere with the surgeonŌĆÖs view during surgery. Since these optical limitations make surgeons hesitate during macular surgery, a previous study comparing the operation time between monofocal IOL and multifocal IOL showed that eyes with multifocal IOLs spend more time in macular surgery for ERM and/or ILM peeling than eyes with monofocal IOL [14]. Patel et al. [15] reported that combined PPV with multifocal IOL can increase the risk of retinal break. Altun [16] also reported an increased risk of iatrogenic retinal break in eyes with multifocal IOL. However, according to our experience with the Eyhance IOL, there was no distortion of the surgical field via IOL, not only in membrane peeling but also in shaving the peripheral vitreous (Supplementary Video 1).
An important factor that determines the success of cataract surgery is the prediction of the refractive power. The clinical importance of refractory power after cataract surgery is most prominent in the implantation of multifocal IOLs. If postoperative refractory power does not achieve emmetropia, it results in patient dissatisfaction with blurred vision and halos [17,18]. The axial length cannot be detected precisely in several retinal disorders, such as VH and macula-off retinal detachment [19]. Moreover, there can be myopic shift after phacovitrectomy [20]. For these reasons, multifocal IOL implantation has been hesitated in patients who require combined cataract and vitreoretinal surgery. The target refraction was emmetropia, but the SEs of the two patients with macula-off RRD were ŌłÆ1.75. This may be caused by incorrect measurement of axial length due to the subretinal fluid in macular area. If this situation occurred in patients with multifocal IOL, the patients would experience discomfort, such as blurred vision and compromised contrast sensitivity. Although the postoperative refractory power of these patients did not achieve emmetropia, the Eyhance IOL satisfied the patients with their ability to preserve intermediate vision and contrast sensitivity. Unsal and Sabur [8] also found that Eyhance IOL was more forgiving to residual refractive errors than standard monofocal IOL.
A major limitation of our study is that it is not a complete comparative study of specific retinal disease entities. The implementation of a patient satisfaction questionnaire or glare test could have potentially demonstrated the advantage of the new monofocal IOL in terms of photic phenomena. However, it is meaningful that we applied this novel IOL in combined cataract and vitreoretinal surgery for various retinal diseases and demonstrated the usefulness of Eyhance IOL in patients with retinal diseases. A comparative study with multifocal IOLs in specific retinal disease is required to definitively address the advantage of this new monofocal IOL. Furthermore, because of the recent release of the new IOL, the study period was too short, and the number of patients included in our study was small. Therefore, the differences in visual acuity and contrast sensitivity according to the types of tamponade, causes of VH, or underlying macular pathology could not be compared statistically.
Visual acuity at intermediate distances is vital for common daily life, such as walking upstairs and downstairs, which has become more important because of the increased use of electronic devices, including tablets, and computers. The growing importance of intermediate vision has led to a desire for an IOL that improves visual acuity at far as well as intermediate distance. Despite not being as expensive as the premium multifocal IOLs, Eyhance IOL is able to provide an effective option in combined cataract and vitrectomy surgery for both patients and surgeons regarding improvement of intermediate and distance visual acuity preserving contrast sensitivity and better visualization of the fundus during surgery. Eyhance IOL will provide retinal patients who undergo combined cataract and vitreoretinal surgery with an alternative to monofocal IOL, which might offer improved quality of vision for daily performance.
Supplementary Materials
Supplementary materials are available from https://doi.org/10.334/kjo.2023.0056.
References
3. Kamath GG, Prasad S, Danson A, Phillips RP. Visual outcome with the array multifocal intraocular lens in patients with concurrent eye disease. J Cataract Refract Surg 2000;26:576-81.


4. Gayton JL, Mackool RJ, Ernest PH, et al. Implantation of multifocal intraocular lenses using a magnification strategy in cataractous eyes with age-related macular degeneration. J Cataract Refract Surg 2012;38:415-8.


5. Sokol S, Moskowitz A, Skarf B, et al. Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol 1985;103:51-4.


6. Tognetto D, Cecchini P, Giglio R, Turco G. Surface profiles of new-generation IOLs with improved intermediate vision. J Cataract Refract Surg 2020;46:902-6.


7. Mencucci R, Cennamo M, Venturi D, et al. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: preliminary results. J Cataract Refract Surg 2020;46:378-87.


8. Unsal U, Sabur H. Comparison of new monofocal innovative and standard monofocal intraocular lens after phacoemulsification. Int Ophthalmol 2021;41:273-82.



9. Auffarth GU, Gerl M, Tsai L, et al. Clinical evaluation of a new monofocal IOL with enhanced intermediate function in patients with cataract. J Cataract Refract Surg 2021;47:184-91.


10. Cinar E, Bolu H, Erbakan G, et al. Vision outcomes with a new monofocal IOL. Int Ophthalmol 2021;41:491-8.



11. Lopes D, Loureiro T, Carreira R, et al. Comparative evaluation of visual outcomes after bilateral implantation of an advanced or conventional monofocal intraocular lens. Eur J Ophthalmol 2022;32:229-34.



12. Aptel F, Colin C, Kaderli S, et al. Management of postoperative inflammation after cataract and complex ocular surgeries: a systematic review and Delphi survey. Br J Ophthalmol 2017;101:1451-60.

13. Vega F, Millan MS, Gil MA, Garzon N. Optical performance of a monofocal intraocular lens designed to extend depth of focus. J Refract Surg 2020;36:625-32.


14. Cao K, Friedman DS, Jin S, et al. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Surv Ophthalmol 2019;64:647-58.


15. Patel SB, Snyder ME, Riemann CD, et al. Combined phacoemulsification surgery with multifocal intraocular lens implantation and pars plana vitrectomy for symptomatic vitreous opacities. Retin Cases Brief Rep 2021;15:724-9.


16. Altun A. Comparing the effect of monofocal and multifocal intraocular lenses on macular surgery. J Ophthalmol 2020;2020:1375298.




17. Gibbons A, Ali TK, Waren DP, Donaldson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin Ophthalmol 2016;10:1965-70.




18. de Vries NE, Webers CA, Touwslager WR, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg 2011;37:859-65.


Fig.┬Ā1
Graphs presenting the contrast sensitivities of patients and controls under (A) photopic condition and (B) mesopic condition. cpd = cycles per degree.
Table┬Ā1
Preoperative characteristics of the patients in the two intraocular lens groups
| Characteristic | Eyhance ICB00* group (n = 33) | Tecnis ZCB00* group (n = 40) | p-valueŌĆĀ |
|---|---|---|---|
| Age (yr) | 59.42 ┬▒ 8.55 | 62.40 ┬▒ 13.39 | 0.953 |
| Sex | 0.904 | ||
| ŌĆāMale | 12 (36.4) | 14 (35.0) | |
| ŌĆāFemale | 21 (63.6) | 26 (65.0) | |
| Spherical equivalent (D) | 1.04 ┬▒ 4.61 | 1.22 ┬▒ 5.30 | 0.862 |
| Cylinder (D) | ŌłÆ0.66 ┬▒ 0.36 | ŌłÆ0.63 ┬▒ 0.32 | 0.841 |
| Axial length (mm) | 23.91 ┬▒ 1.28 | 24.12 ┬▒ 1.83 | 0.694 |
| Preoperative BCDVA (logMAR) | 0.94 ┬▒ 0.93 | 0.93 ┬▒ 0.83 | 0.020 |
| Preoperative CMT (╬╝m) | 402.10 ┬▒ 167.79 | 354.92 ┬▒ 139.48 | 0.711 |
| Main reason for vitrectomy | 0.511 | ||
| ŌĆāVitreous hemorrhage | 12 (36.4) | 12 (30.0) | |
| ŌĆāRhegmatogenous retinal detachment | 4 (12.1) | 9 (22.5) | |
| ŌĆāOpacity | 3 (9.1) | 9 (22.5) | |
| ŌĆāEpiretinal membrane | 9 (27.3) | 8 (20.0) | |
| ŌĆāMacular hole | 3 (9.1) | 2 (5.0) | |
| ŌĆāVitreomacular traction | 2 (6.1) | 0 (0) |
Table┬Ā2
Postoperative characteristics of the patients in the two intraocular lens groups
| Characteristic | Eyhance ICB00* group (n = 33) | Tecnis ZCB00* group (n = 40) | p-valueŌĆĀ |
|---|---|---|---|
| Operation time (min) | 49.39 ┬▒ 9.13 | 48.53 ┬▒ 20.66 | 0.141 |
| Postoperative CMT (╬╝m) | 301.33 ┬▒ 80.56 | 325.60 ┬▒ 83.32 | 0.017 |
| Postoperative SE (D) | ŌłÆ0.35 ┬▒ 0.49 | ŌłÆ0.47 ┬▒ 0.52 | 0.963 |
| Postoperative BCDVA (logMAR) | 0.13 ┬▒ 0.14 | 0.20 ┬▒ 0.25 | 0.295 |
| Postoperative UCDVA (logMAR) | 0.19 ┬▒ 0.14 | 0.25 ┬▒ 0.27 | 0.832 |
| Postoperative UCIVA (logMAR) | 0.29 ┬▒ 0.14 | 0.49 ┬▒ 0.28 | 0.001 |
| Postoperative UCNVA (logMAR) | 0.54 ┬▒ 0.20 | 0.62 ┬▒ 0.28 | 0.267 |
| Postoperative UCIVA:UCDVA | 0.81 ┬▒ 0.11 | 0.66 ┬▒ 0.20 | <0.001 |
| Postoperative UCNVA:UCDVA | 0.47 ┬▒ 0.12 | 0.45 ┬▒ 0.19 | 0.239 |
CMT = central macular thickness; SE = spherical equivalent; D = diopters; BCDVA = best-corrected distant visual acuity; logMAR = logarithm of the minimum angle of resolution; UCDVA = uncorrected distant visual acuity; UCIVA = uncorrected intermediate visual acuity; UCNVA = uncorrected near visual acuity.
Table┬Ā3
Univariate and multivariate analysis of postoperative UCIVA in the Eyhance ICB00* group
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print




