 |
 |
| Korean J Ophthalmol > Volume 37(5); 2023 > Article |
|
Abstract
Purpose
Although the popularity of Descemet membrane endothelial keratoplasty (DMEK) is increased, there is still few clinical studies in Korea. In this study, we aimed to report the initial clinical outcomes of DMEK in patients followed up for more than 6 months.
Methods
A total of 96 eyes that underwent DMEK by a single surgeon for Fuchs endothelial corneal dystrophy, pseudophakic bullous keratopathy, or other indications were evaluated for best-corrected visual acuity (BCVA), endothelial cell density (ECD), central corneal thickness (CCT), postoperative complications, and graft survival.
Results
The postoperative BCVA significantly increased compared to the preoperative BCVA by 59.4% (1.00 ± 0.77 logarithm of the minimum angle of resolution vs. 0.67 ± 0.76 logarithm of the minimum angle of resolution, p < 0.001). The average preoperative ECD was 754 ± 382 cells/mm2, increasing to 1,333 ± 562 cells/mm2 at 3 months (76.8%, p < 0.001), 1,334 ± 632 cells/mm2 at 6 months (76.9%, p < 0.001), 1,121 ± 474 cells/mm2 at 12 months (48.7%, p = 0.024), and 972 ± 458 cells/mm2 at 24 months postoperatively (28.9%, p = 0.445). Compared to 3 months, the ECD declined by 15.9% at 12 months (p = 0.009) and 27.1% at 24 months postoperatively (p = 0.158). The average CCT was 675 ± 113 μm preoperatively, decreasing to 581 ± 102, 574 ± 101, and 594 ± 94 μm at 6, 12, and 24 months after DMEK, respectively (p < 0.001 between all follow-up time points). Allograft rejection was detected in three (3.1%) and 14 eyes (14.6%) underwent retransplantation at an average of 10.1 ± 8.4 months after DMEK.
The introduction of endothelial keratoplasty (EK) has revolutionized corneal transplantation for several decades. Penetrating keratoplasty (PKP) was replaced with Descemet stripping automated EK (DSAEK), which involves the selective removal of the dysfunctional endothelium, followed by the transplantation of donor corneal endothelium, Descemet membrane (DM), and a portion of the donor corneal stroma. Subsequently, DSAEK was replaced with DMEK. DMEK involves the selective transplantation of a cell sheet, which is composed of corneal endothelium and DM, and is the most common type of corneal transplantation in the Western world [1,2].
Surgeons skilled in DMEK have been increasing with its increasing popularity globally. However, there are limited case reports [3,4], and long-term observational surgical reports have not yet been published in Korea. Previous publications and case reports of DMEK in Korea have involved a limited number of cases and provided short-term results [5]. Moreover, no studies have compared the clinical outcomes of DMEK and DSAEK. Considering the global popularity of EK, there is the need to explore its indications and outcomes in Korea. In light of this, the primary purpose of this study was to report the initial outcomes of EK for cases followed up for more than 6 months, including 96 cases of DMEK.
This study was approved by the Institutional Review Board of Yonsei University College of Medicine (No. 3-2023-0050). The requirement for informed consent was waived due to the retrospective nature of the study. The authors adhered to the tenets of the Declaration of Helsinki throughout the study.
This was a single-centered, retrospective case series of patients who underwent DMEK performed by a single surgeon from January 2017 to July 2022. A total of 96 eyes that underwent DMEK for Fuchs endothelial corneal dystrophy (FECD), Pseudophakic bullous keratopathy (PBK), or other indications (including phakic intraocular lens, corneal decompensation due to trauma or infection, bullous keratopathy due to glaucoma, previous PKP) by a single surgeon were included. About the exclusion, as this report is designed as retrospectively, we excluded the cases which has incomplete data set and follow-up loss in 6 months.
The data for the study included the preoperative and postoperative best-corrected visual acuity (BCVA), endothelial cell density (ECD), central corneal thickness (CCT), and postoperative complication. Visual acuities evaluated by Snellen charts were converted to logarithm of the minimal angle of resolution (logMAR) values for analysis. At each visit, the patients underwent slit-lamp examination, tonometry, and specular microscopic examinations. All the patients underwent anterior segment optical coherence tomography (CASIA, Tomey Corp) at every visit during the first month, followed by every 3 months for at least a year after surgery. Microsoft Excel (Microsoft Corp), GraphPad Prism 8.0 (GraphPad Software), and IBM SPSS ver. 26.0 (IBM Corp) were used to analyze the data and plot graphs. Descriptive statistics are reported as mean ± standard deviation for continuous variables and number and percentage for categorical variables. Wilcoxon signed-rank test was used to assess the differences at the various follow-up points. A p-value of 0.05 denoted statistical significance.
Of the 96 eyes that underwent DMEK, 17 received local anesthesia with topical 4% lidocaine. General anesthesia was administered for the other 79 cases. The choice of anesthesia was based on patient preference. All the donor tissues were provided by EverSight Eye Bank. They were preloaded in a DORC tube (DORC Dutch Ophthalmic Research Center), with S stamps with sizes of 7.5 to 8.5 mm, and they had ECDs of > 2,000 mm2.
The host cornea was lightly ink-marked with an 8.5-mm caliper tip as a template for Descemet striping. Two horizontal incisions for paracentesis were made at 3 o’clock from the main incision with a 0.9-mm paracentesis knife. A 26-gauge needle was introduced into the anterior chamber and air injection was performed. A reverse Sinsky hoop was introduced through the tunnel using a 26-gauge needle and the DM was stripped out with the previous ink-marked margin as a guide. After the Descemetorhexis, a DMEK graft was inserted using a glass pipette (DORC Dutch Ophthalmic Research Center). The main incision wound was sutured with 10-0 Nylon (Ethicon Inc). A tapping technique was used for unfolding. Subsequently, the anterior chamber was filled with air or 20% diluted sulfur hexafluoride gas (almost all cases) when the graft was unrolled in the intended area. We considered a full duration of inflation of 30 minutes. A balanced salt solution was used to pressurize the eye as needed.
The following medications were used after surgical techniques. One percent prednisolone (PredForte, Allergan) and 0.5% moxifloxacin eye drops (Vigamox, Novartis Pharma AG) were applied six times a day for a week and three times a day for the second week. Both eyedrops were kept for 2 more weeks. Systemic antibiotics and glucocorticoids were administered until 7 days after surgery.
At 6, 12, and 24 months after DMEK, 79, 63, and 12 eyes, respectively, were still available for analysis. The demographic data of the included patients are summarized in Table 1.
Preoperatively, the BCVA was ≥20 / 40 for 24 eyes (25.0%), ≥20 / 25 for 15 eyes (15.7%), and ≥20 / 20 for nine eyes (9.4%). Among the postoperative, BCVA was ≥20 / 40 for 48 eyes (50.0%), ≥20 / 25 for 30 eyes (31.3%), and ≥20/20 for 17 eyes (17.7%).
The postoperative BCVA significantly increased compared to the preoperative BCVA by 59.4% (n = 57; 1.00 ± 0.77 logMAR vs. 0.67 ± 0.76 logMAR; p < 0.001, Wilcoxon signed-rank test) (Fig. 1A). The proportion of eyes with ≥1-line improvement was 5.2% (3 of 57), ≥2-line improvement was 12.2% (7 of 57), ≥3-line improvement was 17.5% (10 of 57), and ≥4-line improvement was 64.9% (37 of 57) (Fig. 1B).
The Wilcoxon signed-rank test evaluated the ECD change. The average preoperative ECD was 754 ± 382 cells/mm2, increasing to 1,333 ± 562 cells/mm2 at 3 months (76.8%, p < 0.001), 1,334 ± 632 cells/mm2 at 6 months (76.9%, p < 0.001), 1,121 ± 474 cells/mm2 at 12 months (48.7%, p = 0.024), and 972 ± 458 cells/mm2 at 24 months postoperatively (28.9%, p = 0.445) (Fig. 2). Compared to 3 months, the ECD declined by 15.9% at 12months ( p = 0.009), 27.1% at 24 months ( p = 0.158).
Meanwhile, the average preoperative donor ECD was 2,907 ± 211 cells/mm2, decreasing to 1,334 ± 632 cells/mm2 at 6 months, 1,121 ± 474 cells/mm2 at 12 months, and 972 ± 458 cells/mm2 at 24 months postoperatively. These corresponded to ECD declines of 54.1%, 61.5%, and 66.6%, respectively, from the preoperative values ( p < 0.001 for all follow-up time points, Wilcoxon signed-rank test). Of the 12 eyes that had ECDs of <800 cells/mm2 at 12 months postoperatively (mean, 696 ± 179 cells/mm2; median, 662 cells/mm2), four were still available after 3 years of follow-up (mean, 792 ± 74 cells/mm2; median, 815 cells/mm2).
The average preoperative CCT was 675 ± 113 μm, and it decreased to 581 ± 102, 574 ± 101, and 594 ± 94 μm at 6, 12, and 24 months after DMEK, respectively. The CCT values were significantly reduced at 3 (data not shown), 6, 12, and 24 months after surgery ( p < 0.001 for all the follow-up time points, Wilcoxon signed-rank test) (Fig. 3).
All the postoperative complications during the follow-up are summarized in Table 2. Graft detachment and corneal swelling were found at day after surgery, and they did not recover until a month despite a rebubbling procedure. At 1 week postoperatively, 24 eyes (25.0%) underwent a rebubbling procedure. Among them, minor graft detachment was detected in 16 eyes (16.7%), and a major detachment was observed in eight eyes (8.3%). Furthermore, seven eyes (7.3%) underwent a rebubbling procedure more than twice. Allograft rejection was detected in three eyes (3.1%) and was reversed by both systemic and topical steroid treatment. However, all eyes eventually had graft failure.
The overall graft survival probability did not incorporate primary graft failure (n = 2), immunological rejection (n = 3), severe cornea edema (>650 μm) or uncheckable ECD at 12 months after surgery (n = 19), and corneal bacterial infection (n = 0). Fourteen eyes (14.6%) underwent retransplantation at an average of 10.1 ± 8.4 months after DMEK. The indications for retransplantation were graft detachment (n = 2) or secondary graft failure (n = 12). All retransplantations were performed within 3 months after diagnosis.
The number of cases of keratoplasty has been increasing in Korea each year, although there are still no annually reported official statistical data. Compared with Western countries, there are several factors to consider when performing keratoplasty in Korea [5,6]. The first is that keratoplasty requires an imported donor, which is associated with a time lag of approximately 7 to 8 days from the preparation of the donor cornea to the surgery [7]. Second, EK has increased in popularity; however, PKP is still the most popular in Korea. Lastly, national insurance does not cover the expense of an imported donor cornea. Therefore, the medical expense and economic burden for keratoplasty, especially DMEK, is much higher in Korea than in other countries. This is a major big huddle, and doctors may be hesitant to perform DMEK. This may explain why DMEK surgery has not been popularized as rapidly and reports on the results of DMEK published in Korea have been limited. In 2015, a limited case series for DMEK was published in Korea [8]. Ten eyes underwent the procedure, and eight demonstrated excellent visual recovery with acceptable ECD (two cases resulted in primary failure). The other recent report on DMEK involved a Korean patient. Jung et al. [5] compared the endothelium-in and endothelium-out techniques on precut tissue for a relatively large number of cases (32 eyes) and found comparable outcomes. ECD had decreased by more than 50% from the preoperative value at 12 months postoperatively.
The graft survival rate was 75% (72 eyes) at 12 months after surgery. This is relatively lower than the survival rates in other reports [9-12]. Recently, Birbal et al. [9] and Vasiliauskaite et al. [10] reported 10- and 5-year survival rates of 79% and 90%, respectively. However, the study included 94% of FECD cases with only 2% of PBK cases. Our study population was more diverse. There were 19.8% of FECD cases, 36.5% of PBK cases, and 43.8% of others (including phakic intraocular lens, corneal decompensation due to trauma or infection). Unlike other studies, PBK accounted for most of the cases included in this study; these cases also had a history of glaucoma surgery or trans pars plana vitrectomy; 29.2% of all the patients had been diagnosed with glaucoma. Therefore, the results may be quite acceptable, given that this is the first experience of the surgeon in caring for 96 consecutive cases.
Besides the surgical technique and preoperative conditions, another critical influencing factor for graft survival may be graft detachment after DMEK. It is well-known that DMEK is negatively affected by graft detachment [9,13]. Graft detachment was assessed with anterior optical coherence tomography postoperatively, and the rate was high in this study at 25.0% (24 cases). Previous studies have reported graft detachment and rebubbling rates of 5% to 11% for FECD cases [9,11,12,14,15]. As mentioned above, the dominance of PBK for our cases and the use of preloaded imported grafts may explain the higher detachment rate in this study. The delay between the preparation of corneal tissue overseas and the surgery (usually 6 to 8 days) may have affected endothelial function [7,16]. We could assume that the transport temperature of preloaded grafts and pH changes of storage media (Optisol-GS) can have enough effects on the ECD and cell viability during the delay of 6 to 8 days. Meanwhile, the cases without graft detachment showed higher ECD and better graft survival (77.8% of graft survival vs. 8.6% in repeated graft detachment).
The outcomes in this study were worse than previously reported. Only six cases (6.3%) maintained BCVA of 20 / 25 or better for up to 24 months after DMEK. These results seem to not corroborate the findings of previous studies that higher visual acuities within the first months after DMEK were also maintained for a longer term [9,11,17-19]. This study included cases of vitrectomy, iris trauma and damage, or advanced glaucoma, and the surgical results were not as optimal. However, visual acuity improved for 57 cases (59.4%) after surgery; 37 cases (38.5%) had improvements of more than four lines. This may imply the DMEK can result in acceptable long-term improvements in BCVA with appropriate preoperative preparations and surgical design and a careful surgical technique.
We found significantly higher ECD declines during the early postoperative period than what was suggested by the donor cornea data. Compared with other reports, which showed approximately 20% to 30% declines in the ECD, more than 50% of the ECD was reduced from the values on the donor information sheet at 1 month postoperatively. The heterogeneity of the cases may have caused the significant loss of ECD during the early postoperative period. However, the recently published Korean DMEK data also showed ECD losses similar to what we observed [5]. We could not find the cause of this significant reduction in ECDs. The time lag between the preparation of corneal tissue overseas and the surgery may have resulted in the differences between the donor data and early postoperative specular microscopy data [7]. Therefore, we have to reduce the delay between tissue preparation and surgery, as much as possible, to facilitate the best surgical outcomes with longer graft survival. In this reason, to evaluate the outcome of DMEK itself, it may be more reasonable to set the initial value at 3 months after DMEK rather than donor ECD or immediately after DMEK. Furthermore, to achieve accurate ECD, we measured the ECD at five different points (up, down, left, right, and front gaze) and calculated the mean values.
The limitations of this study are the learning curve of the surgeon and the heterogeneity of the cases. First, the learning curve of the surgeon had a significant impact on the clinical outcomes; therefore, the results of this study should be interpreted carefully. Second, the sizes of the DMEK grafts were not uniform. The 8.0-mm precut and preloaded tissues were used for most cases, but 7.5- or 8.5-mm sized tissues were also used. The total number of corneal endothelial cells was significantly increased by the size of the graft, and this should be considered for the evaluation of surgical outcomes in future studies. Last, the variable preoperative conditions may have affected the surgical outcomes.
DMEK has acceptable outcomes with low complication rates, even for vitrectomized eyes and those with advanced glaucoma. Domestic DMEK donors are rare in Korea, and using an imported preloaded graft is a safe option against corneal endothelial failure several years after surgery. Further well-controlled studies involving more cases and long-term follow should be conducted.
References
1. Price MO, Gupta P, Lass J, Price FW Jr. EK (DLEK, DSEK, DMEK): new frontier in cornea surgery. Annu Rev Vis Sci 2017;3:69-90.


2. Godinho JV, Mian SI. Update on Descemet membrane endothelial keratoplasty. Curr Opin Ophthalmol 2019;30:271-4.


3. Yum HR, Kim MS, Kim EC. Retrocorneal membrane after Descemet membrane endothelial keratoplasty. Cornea 2013;32:1288-90.


4. Son WY, Ha MJ, Whang WJ, et al. Descemet membrane endothelial keratoplasty after penetrating keratoplasty graft failure. J Korean Ophthalmol Soc 2021;62:848-54.


5. Jung YH, Yoon CH, Kim MK. Clinical outcome of Descemet membrane endothelial keratoplasty (DMEK) with imported donor corneas in eyes of Asian patients: endothelium-in versus endothelium-out method. PLoS One 2022;17:e0270037.



6. Hayashi T, Oyakawa I, Kato N. Techniques for learning descemet membrane endothelial keratoplasty for eyes of Asian patients with shallow anterior chamber. Cornea 2017;36:390-3.



7. Newman LR, DeMill DL, Zeidenweber DA, et al. Preloaded Descemet membrane endothelial keratoplasty donor tissue: surgical technique and early clinical results. Cornea 2018;37:981-6.


8. Han GL, Hyun J, Lim DH, et al. Clinical outcomes of Descemet’s membrane endothelial keratoplasty: a 1-year retrospective study. J Korean Ophthalmol Soc 2015;56:1489-96.

9. Birbal RS, Ni Dhubhghaill S, Bourgonje VJ, et al. Five-year graft survival and clinical outcomes of 500 consecutive cases after Descemet membrane endothelial keratoplasty. Cornea 2020;39:290-7.


10. Vasiliauskaite I, Oellerich S, Ham L, et al. Descemet membrane endothelial keratoplasty: ten-year graft survival and clinical outcomes. Am J Ophthalmol 2020;217:114-20.


11. Price MO, Giebel AW, Fairchild KM, Price FW Jr. Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009;116:2361-8.


12. Guerra FP, Anshu A, Price MO, et al. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology 2011;118:2368-73.


13. Gerber-Hollbach N, Baydoun L, Lopez EF, et al. Clinical outcome of rebubbling for graft detachment after Descemet membrane endothelial keratoplasty. Cornea 2017;36:771-6.


14. Chaurasia S, Price FW Jr, Gunderson L, Price MO. Descemet’s membrane endothelial keratoplasty: clinical results of single versus triple procedures (combined with cataract surgery). Ophthalmology 2014;121:454-8.


15. Feng MT, Price MO, Miller JM, Price FW Jr. Air reinjection and endothelial cell density in Descemet membrane endothelial keratoplasty: five-year follow-up. J Cataract Refract Surg 2014;40:1116-21.


16. Chen C, Solar SJ, Lohmeier J, et al. Viability of preloaded Descemet membrane endothelial keratoplasty grafts with 96-hour shipment. BMJ Open Ophthalmol 2021;6:e000679.



17. Ham L, Dapena I, Liarakos VS, et al. Midterm results of Descemet membrane endothelial keratoplasty: 4 to 7 years clinical outcome. Am J Ophthalmol 2016;171:113-21.


Fig. 1
(A) Preoperative and postoperative best-corrected visual acuity (BCVA) in logarithm of the minimal angle of resolution (logMAR). Values are presented as mean ± standard deviation. (B) Line improvement of BCVA in logMAR (n = 57). *Wilcoxon signed-rank test. A p-value of <0.05 was considered statistically significant.
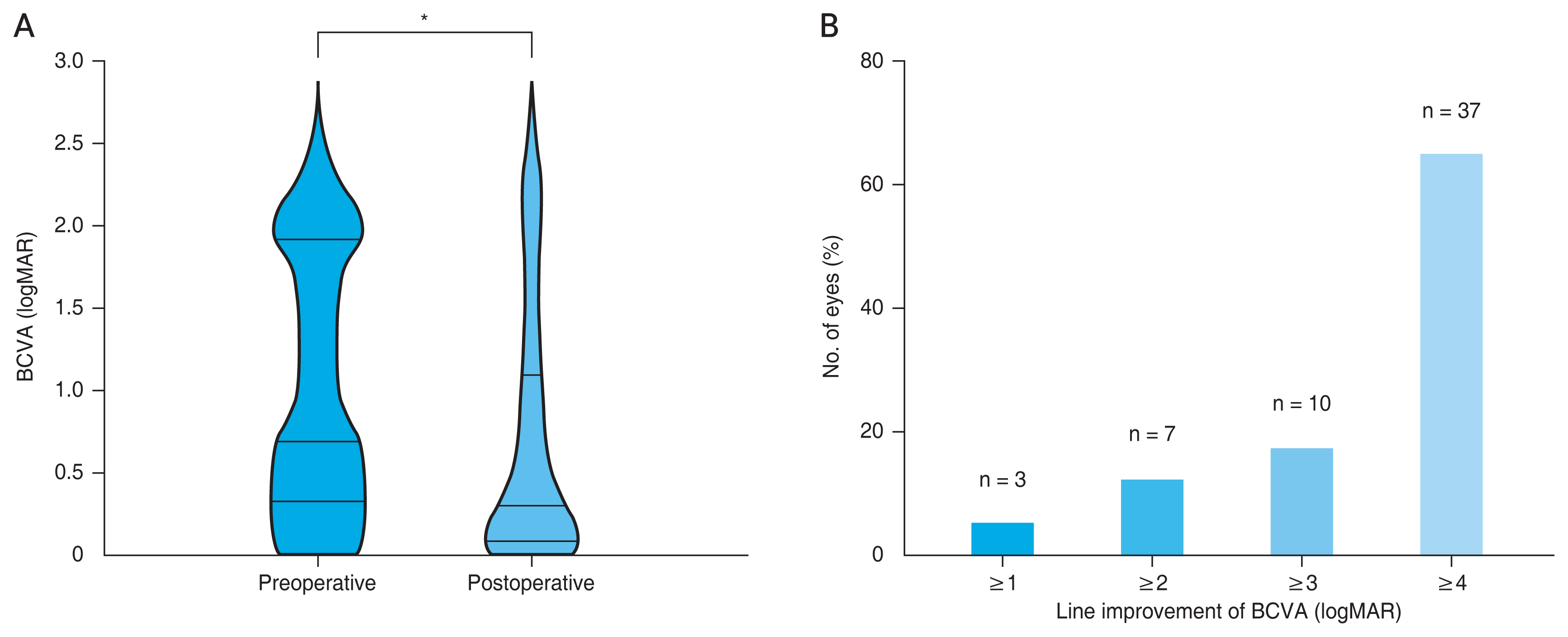
Fig. 2
Endothelial cell density (ECD) for up to 2 years after Descemet membrane endothelial keratoplasty. Mean ECD values are shown (bar indicates standard deviation). NS = no significant difference ( p ≥ 0.05). *Wilcoxon signed-rank test. A p-value of <0.05 was considered statistically significant.
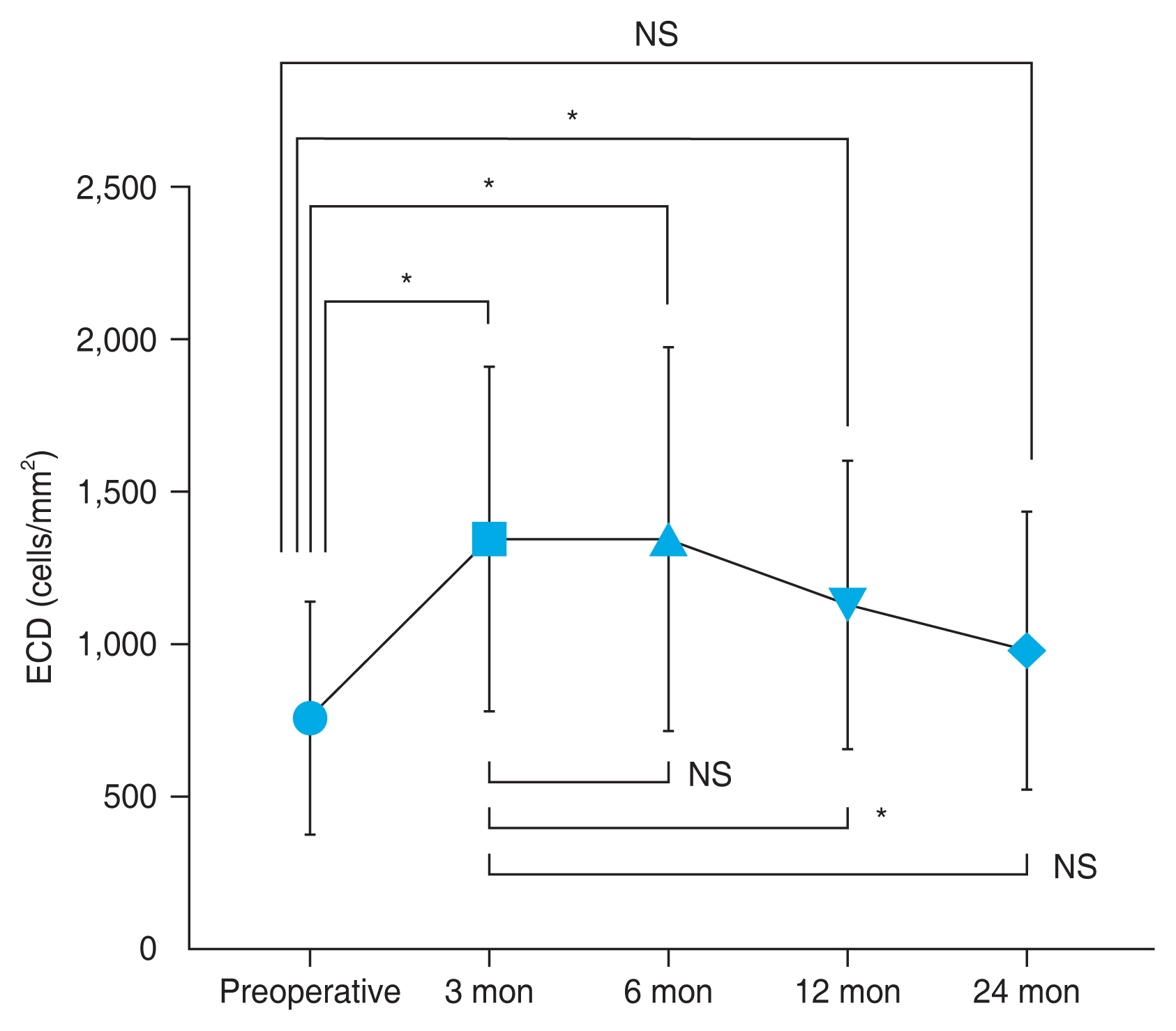
Fig. 3
Central corneal thickness changes after Descemet membrane endothelial keratoplasty. Line graph presents the mean corneal thickness at 6, 12, and 24 months (bar indicates standard deviation). *Wilcoxon signed-rank test. A p-value of <0.05 was considered statistically significant.
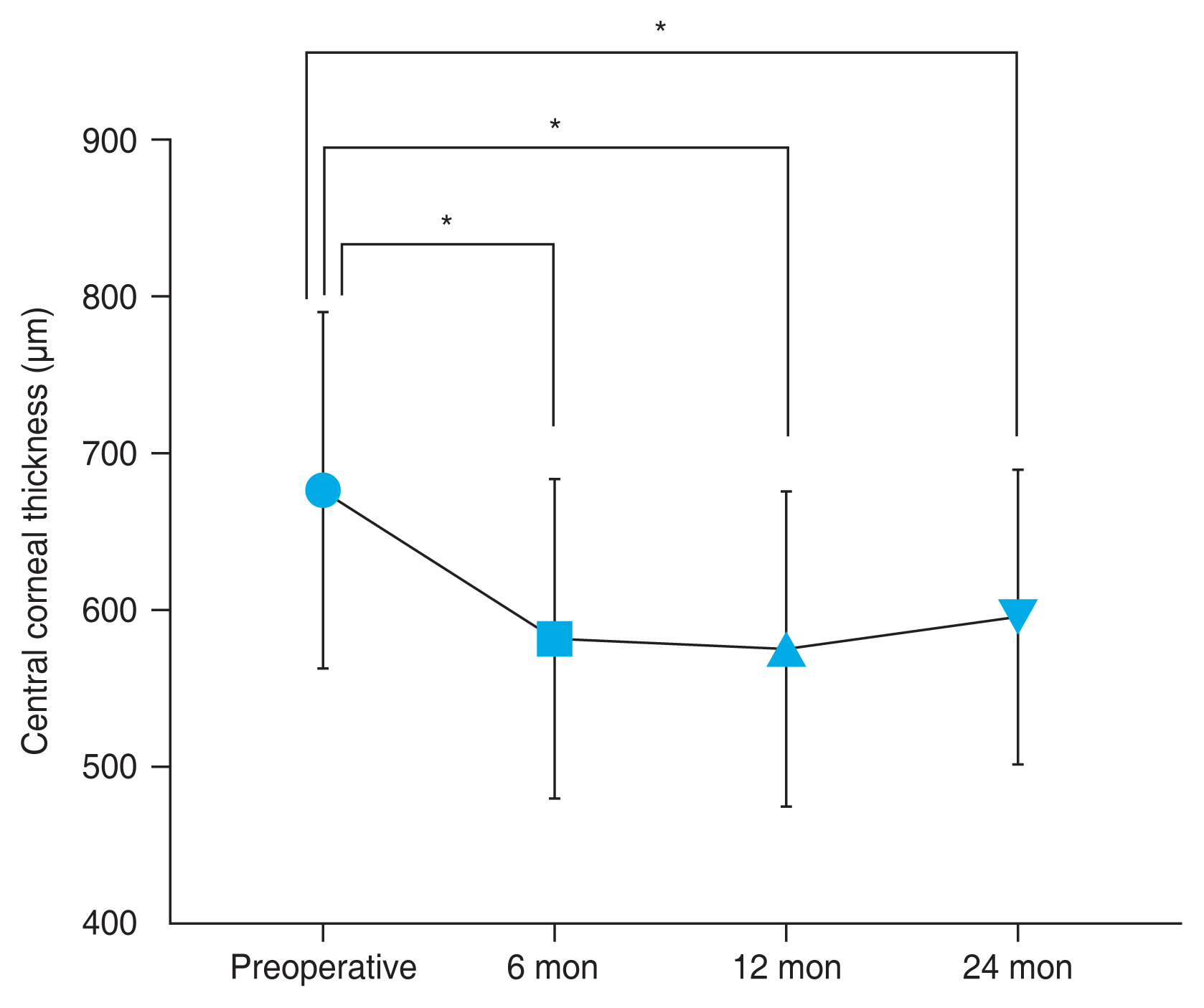
Table 1
Demographics of patients who underwent DMEK
| Demographic | Value (n = 96) |
|---|---|
| Sex | |
| Male | 45 (46.9) |
| Female | 51 (53.1) |
| Age (yr) | 60.0 ± 13.6 |
| Follow-up (day) | 527.6 ± 448.6 |
| Median (range) | 371 (57-1,673) |
| Indication for DMEK | |
| Fuchs endothelial corneal dystrophy | 19 (19.8) |
| Pseudophakic bullous keratopathy | 35 (36.5) |
| Other* | 42(43.8) |
| Diabetes mellitus history | |
| Yes | 16 (16.7) |
| No | 80 (83.3) |
| Glaucoma history | |
| Yes | 28 (29.2) |
| No | 68 (70.8) |
Table 2
Postoperative complications and secondary procedures after DMEK
| Variable | Value (n = 96) |
|---|---|
| Graft detachment* and secondary procedure (rebubbling) | 24 (25.0) |
| Minor detachment (≤1/3 of graft surface area) | 16 (16.7) |
| Major detachment (>1/3 of graft surface area) | 8 (8.3) |
| Graft failure | |
| Primary† | 2 (2.1) |
| Allograft rejection | 3 (3.1) |
| Severe corneal edema (>650 μm) or uncheckable ECD at 12 months after surgery | 19 (19.8) |
| Retransplantation | 14 (14.6) |
| Graft detachment | 2 (2.1) |
| Secondary graft failure‡ | 12 (12.5) |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




