Childhood cataract is a significant cause of childhood blindness globally, particularly in sub-Saharan Africa [
1]. Childhood cataracts may be familial which could present as congenital or developmental cataracts.
Familial cataracts have been reported in literature as far back as 1888 [
2]. In a nationwide Danish study, 23% of congenital cataracts were hereditary and almost all were bilateral and isolated without any additional ocular or systemic abnormalities [
3]. In majority of these, the inheritance pattern was autosomal dominant.
Inheritance in familial cataract is mendelian with high penetrance and autosomal dominance mode is most common. However, there are reports of autosomal recessive and X-linked inheritance pattern [
4-
6].
It has been postulated that the proportion of inherited cataracts is lower in developing countries due to a disproportionately higher risk of cataracts from infectious and other environmental causes [
7]. In spite of this, etiological diagnosis of childhood cataract is still limited in most low-and lower-middle-income countries due to nonavailability or affordability of diagnostics tests. This, therefore, necessitates a high level of clinical acumen to make a near accurate diagnosis based mainly on clinical features.
Early identification and diagnosis can facilitate intervention that is timely and appropriate and in turn ensure that visual outcomes are optimized. With improved access to genetic testing, it has become important to describe varied clinical profiles to improve the awareness of clinicians.
In the absence of genetic testing, a careful pedigree history and systemic examination can help narrow down the probable causes of congenital cataracts [
8]. This has the potential to reduce the burden of blindness from childhood cataract by improving awareness among parents at risk of having a baby with cataract and ensuring early intervention for those affected.
With increasing availability and spread of pediatric ophthalmology practice in Nigeria, it is necessary to generate local data on pediatric ocular conditions including data on familial cataract. With particular reference to familial cataract, there is a dearth of literature from Nigeria, and this may be related to the fact that ocular genetics practice is at a nascent state in Nigeria. Therefore, a review of the pattern of presentation of familial cataract may be useful to guide ophthalmologists in genetic counseling, recurrence risk counseling, choosing effective surgical interventions and guide surveillance of families with familial cataracts.
The aim of this study is to describe the clinical profile, pedigree charting, ocular and systemic comorbidities, and management of children with familial cataracts in the ophthalmology clinic of the University College Hospital Ibadan (Ibadan, Nigeria) over a 5-year period.
Materials and Methods
This study was a retrospective case series of all children aged 0 to 16 years who were diagnosed with familial cataracts at the Pediatric Ophthalmology and Strabismus Clinic, University College Hospital Ibadan (Ibadan, Nigeria) from January 1, 2015, to December 31, 2019. Ethical approval was obtained from the University of Ibadan/University College Hospital Ethical Review Committee (No. UI/EC/23/0005). Informed consent was waived due to the retrospective nature of the study. Patient data were anonymized in this report.
The clinic records of patients with familial cataracts were reviewed. Cases with missing records were excluded. All patients with features suggestive of intrauterine infection, drug exposure, metabolic disorders, or malnutrition were also excluded. Information on demographic data, visual acuity, refractive error, family history, laterality, clinical features, associated ocular and systemic abnormalities, treatment received, and follow-up were retrieved from the case notes.
With regards to the surgical management, children younger than 2 years of age underwent lensectomy, primary posterior capsulotomy, and anterior vitrectomy; while older children had small incision cataract surgery with insertion of a posterior chamber intraocular lens. After surgery, the children were kept on admission for 5 to 7 days as required. Postoperative medications include dexamethasone eyedrops, antibiotics drops and tropicamide (for pseudophakia) or atropine (for aphakia), and dexamethasone ointment at night. Children who had intraocular lenses implanted had oral prednisolone at 1mg/kg alternate day for 2 weeks as well as antacids.
Manifest refraction with a retinoscope was performed; spectacles were dispensed, and amblyopia treatment was initiated to optimize vision. Age-appropriate visual acuity assessment was conducted for all patients at every follow-up visit: at 2 weeks, 1 month, 3-month intervals until the end of the postoperative 1st year, and 6-month intervals thereafter. Visual acuity for children 4 years or younger was done at 3 m while those older than 4 years had assessment at 6 m. Near visual acuity was not routinely assessed. The final visual acuity presented in this report is the visual acuity recorded at the last follow-up visit for each child.
The data was entered into a Microsoft Excel spreadsheet (Microsoft Corp) and IBM SPSS ver. 20 (IBM Corp) was used to perform the analyses. For qualitative variables, the frequencies and percentage proportions were calculated. For quantitative variables, the mean and standard deviations were calculated.
Results
Demographic characteristics
A total of 42 patients with familial cataracts were identified from the records but four were excluded as their medical records were incomplete or missing. This number accounted for 13.77% of the total number of children with pediatric cataracts presenting at the eye clinic within the period. Consequently, the study included 38 probands with familial cataract; the total number of families contributing to this data were 24 nuclear families.
There were 25 male (65.8%) and 13 female patients (34.2%), with a ratio of 1.9:1. The mean age at presentation was 6.30 ± 3.68 years, with a range of 7 months to 13 years. All patients had bilateral involvement, with asymmetric severity in some cases. The mean duration from onset of symptoms to presentation at the hospital was 3.71 ± 3.20 years, with a range of 3 months to 13 years.
The morphological types of cataracts in the study cohort are shown in
Table 1. The four most common types were cerulean (
Fig. 1), partially absorbed, full, and lamellar, accounting for 71.8% of all eyes with cataract. At presentation, 71 eyes of 76 eyes had not undergone any cataract surgery. Five eyes in three patients had been operated in another facility prior to presentation at our clinic, of these eyes three were aphakic, one pseudophakic, and one buphthalmic following complications from cataract surgery in another facility.
A pedigree charting of the 24 nuclear families revealed a history of childhood cataract in either parent in 21 (87.5%), and in at least one sibling in 19 (79.2%) (
Fig. 2).
Ocular and systemic abnormalities
An associated ocular comorbidity was found in 17 probands (44.7%). Nystagmus was the most common ocular association and was present in seven probands (18.4%). Other associated ocular comorbidities are shown in
Table 2. No systemic abnormalities were found in any of the probands.
Treatment modalities
Of the 71 eyes with cataract at presentation, cataract surgery was performed in 67 eyes. Thirty-two children had bilateral cataract surgery, and three had unilateral cataract surgery. Of the four eyes with cataract that did not have cataract surgery during the study period, one patient defaulted before surgery could be performed in either of the two eyes, while the other two patients defaulted before they could have the second eye surgery. Of the two children who had cataract surgery in both eyes prior to presentation, they were prescribed refractive correction for bilateral aphakia. The child who had a unilateral pseudophakia prior to presentation had second eye surgery in our care and received refractive correction subsequently.
Fifty-seven eyes (85.1%) of the 67 eyes that had cataract surgery, underwent small incision cataract surgery and posterior chamber intraocular lens implantation, and 10 eyes (14.9%) underwent lensectomy only. Primary posterior capsulotomy and anterior vitrectomy was performed in 16 eyes (23.9%).
Following surgery, only 19 (54.3%) of 35 children who had surgery had postoperative refraction. Sixteen children (45.7%) did not have refraction measured after cataract surgery as they had stopped clinic attendance before the usual 3 months postoperative period schedule for refraction in pseudophakia in our center.
The distribution of the best-corrected preoperative and postoperative visual acuity of the operated eyes is presented in
Fig. 3A and 3B. There was an increase in the proportion of eyes with best-corrected visual acuity ≥6 / 18 from 9.1% preoperatively to 52.7% at the last follow-up visit. The mean duration of follow-up after surgery was 9.78 ± 8.35 months, median was 7.5 months, with a range of 1 month to 3 years.
Pedigree charting
A pedigree chart was drawn for most of the patients in the study cohort from details of family history. In addition, a few parents and siblings of the probands were available for examination. A total of 17 pedigree charts were available and some of the 24 nuclear families were linked within larger extended families as represented in the pedigree charts (
Fig. 4,
5).
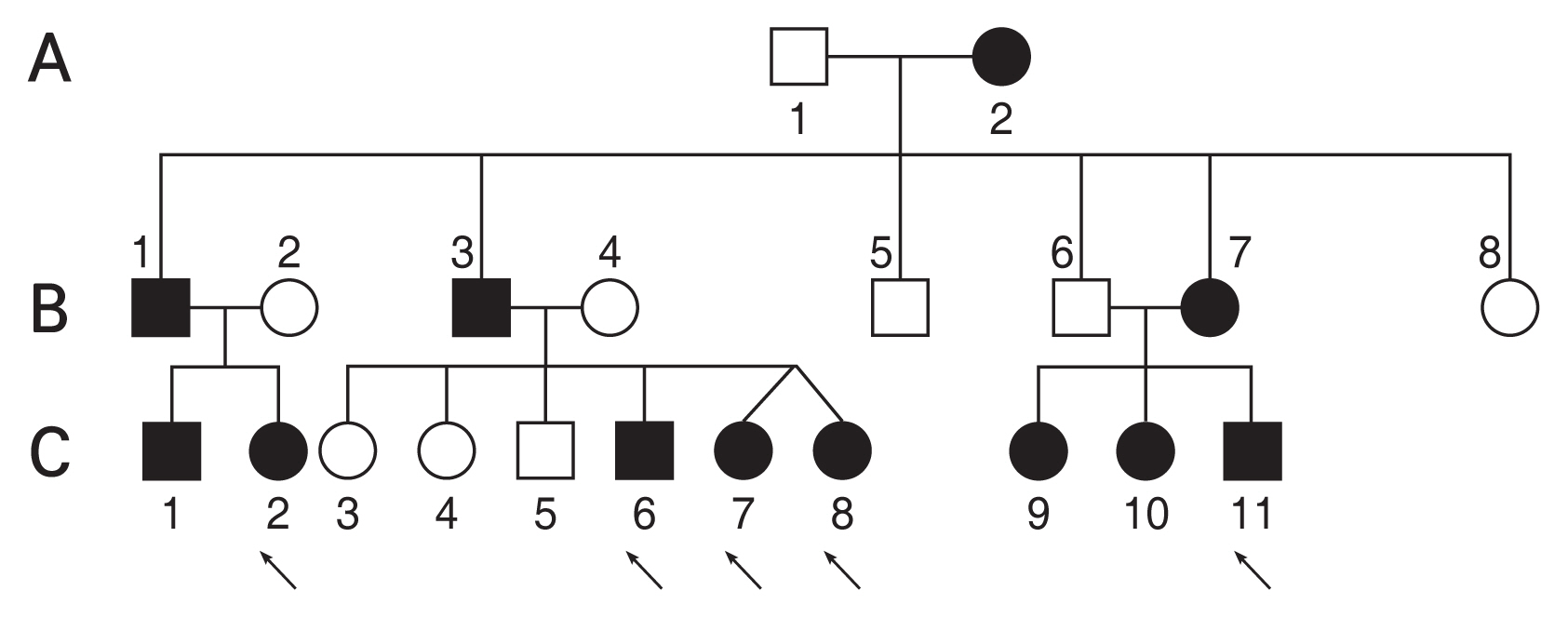
One extended family had eight children (siblings and cousins) in the study cohort (
Fig. 4). All had bilateral cerulean cataracts and they all had cataract surgery. The children were extended family members of three nuclear families in the study cohort and there was a history of childhood cataract or cataract surgery spanning across three generations. Patients from this family included in the study cohort are C1, C2, C6, C7, C8, C9, C10, and C11.
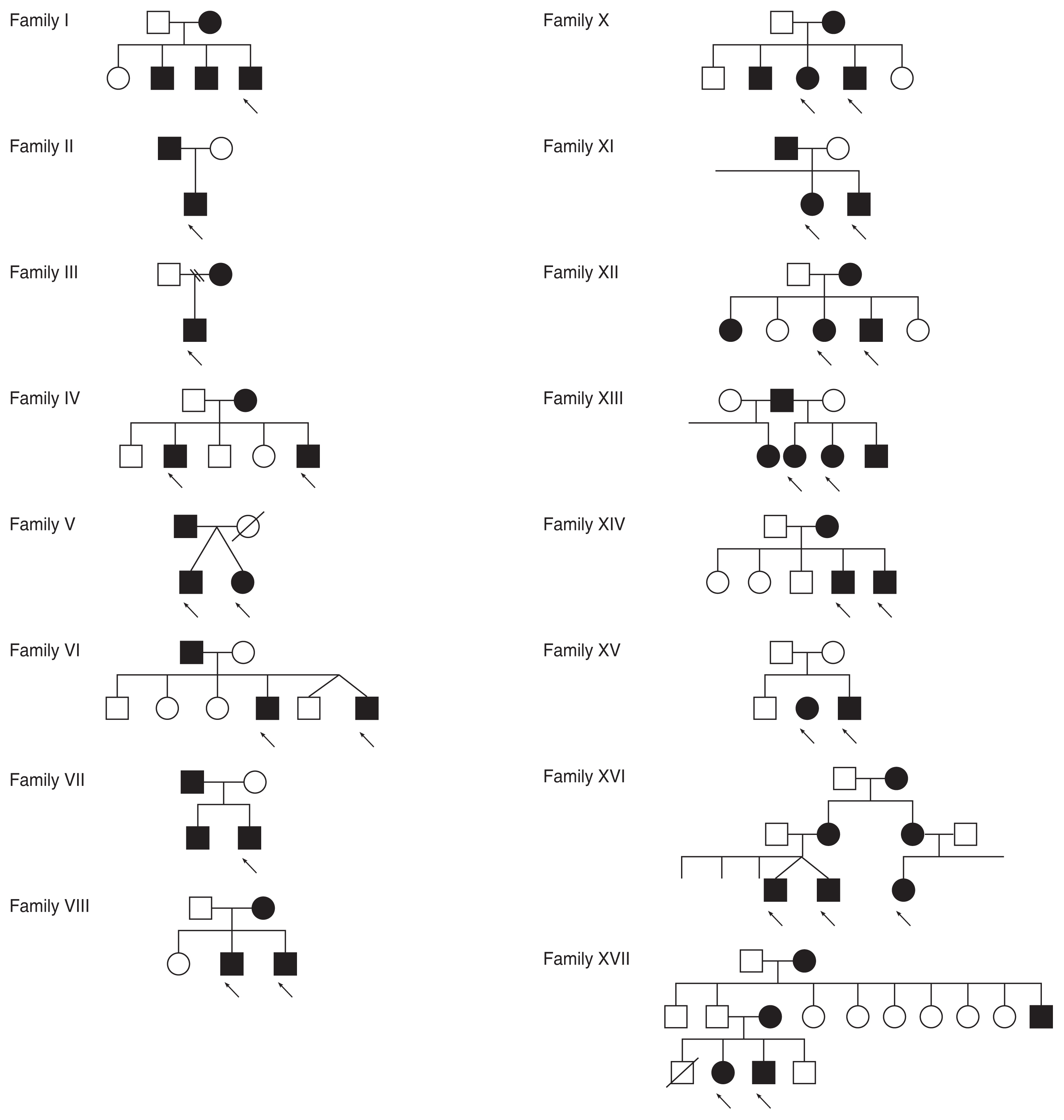
The pedigree charting of other affected families are as depicted in
Fig. 5. In 16 of the pedigree charts, at least one individual was affected in each generation; while in the only chart, in which one generation was skipped, two siblings (a boy and a girl) were affected (family XV).
Discussion
This present study describes the pedigree charting, clinical profile, ocular comorbidities, and management of children with familial cataracts in an indigenous African population. In our environment, case ascertainment for many diseases by population-based active surveillance is still a challenge [
9-
11]. Currently, there is no nationwide screening service for pediatric cataracts. Also, there is no well-established program for routine ocular examination of newborn infants or routine assessment of children either in immunization clinics or during resumption at school. Thus, there is limited capacity to monitor normal visual development or to detect ophthalmic disorders in children.
There were twice as many male patients than female patients presenting with familial cataract. We think this may be an incidental finding as there is no clear-cut sex predilection for familial cataracts except for a few cases of X-linked inheritance which may skew the balance towards males being more likely to be affected. Another possibility is the “gender gap” in seeking care for childhood cataracts in low- and middle-income countries [
12].
Morphologically, cerulean cataract was the most common in this case series. Cerulean cataract also referred to as “blue dot” cataract is an uncommon morphology with characteristic multiple bluish and white opacities mostly in the cortex of the lens with occasional radial central lesions. Coralliform and cerulean cataracts are thought to be different forms of variable expressivity of a single entity [
13]. In the current study, one family had cataracts in three generations. Members of the family from two generations were examined and they all had the cerulean morphology. The genetic mutation responsible for the phenotype in this particular family might be worth studying to detect if it is a novel gene. This reinforces the need for better access to genetic services in our environment in order to aid diagnosis, counseling and treatment.
In a large cohort of 161 patients with autosomal dominant cataracts, phenotypes recognized include polar, nuclear, lamellar, coralliform, cerulean, cortical, and pulverulent [
14]. The cataract phenotype by itself cannot efficiently predict or indicate the affected gene or mutation, since identical cataracts can result from mutations at different loci and may have several inheritance patterns. Also, various cataract types can result even when the same gene is affected, demonstrating clinical heterogeneity [
8].
Other ocular comorbidities identified in these patients could be either ocular associations or could be due to amblyopia. Best-corrected visual acuity was worse than 6 / 60 in about a fifth of the operated eyes. Notwithstanding, about half of the children achieved good vision following surgery. The probable causative factors of this could be late presentation, as the mean duration from onset of symptoms to presentation was 3.71 years. Notably, one patient was brought for care 13 years after visual symptoms were first noticed. Since all the patients had a family history of cataract, it would have been expected that such complaints should have necessitated earlier presentation, but it appears that the opposite was the scenario. This, therefore, buttresses the need to ensure adequate education on awareness and vigilance among families of children with childhood cataract about the possibility of cataract in a sibling or their offspring.
Pedigree charting provides information for counseling such families on the specific risk of inheritance. In our cohort of patients, most of the charts were suggestive of an autosomal dominant mode of inheritance because at least one person was affected in each of the generations represented in those charts. This is in keeping with the reported pattern of inheritance in Caucasian patients [
14]. In one of the families in our study, both parents were not affected; this suggests a recessive pattern of inheritance which is not sex-linked since the two affected siblings were of different sexes. The apparent rarity of autosomal recessive familial cataracts in our population may be because consanguineous marriage is not commonly practiced in West Africa. Recessive inheritance is more likely among the Arabian population [
15], and in societies were consanguineous marriage in common such as in the Middle East, North Africa, and West Asia [
16].
Pedigree charting was apparently easier to achieve in the current study compared to the report by Babalola et al. [
17] from northern Nigeria 20 years ago. They reported a four-generation family with dominantly inherited cataracts associated with recessively inherited sickle cell anemia. They noted then that there were challenges with constructing pedigrees as a result of transportation difficulties and unreliable family history, hence even though many hereditary conditions may be encountered it was difficult to confirm them as such. The challenges encountered then are probably different from current realities. Furthermore, their case report 20 years ago highlighted the need for genetic counseling services in clinical practice in Nigeria but, unfortunately, not much progress has been made. Genetic counseling services are still at the nascent phase in routine clinical care of patients with cataracts.
Cataracts inherited in an autosomal dominant pattern have been diagnosed in utero via the aid of a transvaginal ultrasound as early as 15 postmenstrual weeks [
18]. In pregnancies at risk for familial cataracts, visualization of the fetal eyes and lenses should be part of the routine assessment at fetal anomaly scans [
19]. Fetal anomaly scan is part of the routine care for pregnant women in our center. However, the practice is still not widespread, and it is thus encouraged to assist parents at high risk of having a child with inherited or familial cataract, to prepare better for the care of the child. In 45% of familial cataracts, mutations of crystallin genes have been reported [
20]. The “abnormal lens diagnosis chip,” a genetic testing of about 150 genes compiled into one target-capture chip is now commercially available and can be employed in management of cataracts, ectopia lentis, and microphthalmia [
21]. Genetic testing as well as regular nationwide ocular disease surveillance are not readily accessible in our environment. This is due to limited availability of genetic study tools as well as resources for setting up ocular disease surveillance. Although genetic testing and surveillance facilitate early diagnosis and intervention as well as provision of epidemiologic data, identification of clinical phenotype also has an important role to play in the care of these patients. For instance, even when genetic testing facilities are available, clinical phenotypes are usually the first consideration before subsequent molecular genetic tests are performed [
22].
The study has the following limitations. In view of the financial implications and the limited availability of genetic testing in our setting, we did not carry out gene sequencing studies on our patients. Genetic testing could have aided diagnosis and the counseling of families. Also, we relied mainly on history for the pedigree charting as some parents and siblings were not available for examination. It is possible that some of the subtle forms of cataracts may have been missed.
In conclusion, management of children with pediatric cataracts should include a detailed family history with pedigree charting to identify familial cases with an intention to offer cataract surgery to the affected individuals. The major pattern of inheritance for familial cataracts in our population appears to be the autosomal dominant mode. A novel genetic mutation may be responsible for the cerulean cataract, which was the most common morphological type in this study. However, genetic testing is required to confirm this, particularly in view of the possibility of clinical heterogeneity. Better access to genetic testing and counseling services will enable the delivery of better health care to families with familial cataract.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




