The presence of zonular weakness or inadequate capsular support during cataract surgeries makes implantation of intraocular lenses (IOLs) into the capsular bag difficult or even impossible. In such cases, IOLs must be placed ŌĆ£out of the bagŌĆØ via ciliary sulcus implantation or scleral fixation methods [
1]. Along with the increasing number of cataract surgeries, secondary IOL implantation has become a common procedure for surgical indications such as aphakia and IOL or crystalline lens dislocation [
2]. Although sulcus implantation is usually preferred when capsular support is compromised, scleral fixation is frequently inevitable and essential to provide stability and support in IOL positioning when a continuous curvilinear capsulorrhexis (CCC) margin is not maintained [
3]. A three-piece IOL with posterior angulation and thin, curved haptics makes it suitable for both ciliary sulcus implantation and scleral fixation.
Since the introduction of conventional transscleral fixation of IOLs [
4], there has been a great advance in the evolution of various IOL scleral fixation methods [
1,
2,
4-
11]. Different fixation techniques have their own advantages and disadvantages [
1,
5-
12]. Lee et al. [
9] introduced a novel technique for transscleral fixation of IOLs by winding thread onto haptics without making knots. This method showed good refractive outcomes and stability comparable with those obtained with the conventional method.
A number of sutureless fixation techniques have been developed to avoid suture-related complications such as suture erosion and exposure [
5-
7,
10,
11,
13,
14]. Intrascleral sutureless IOL fixation techniques have also been developed and have gained popularity over the recent years [
5,
15]. Yamane et al. [
7] introduced a new sutureless intrascleral IOL fixation method, in which a haptic is cauterized to form a flange for intrascleral fixation. This flanged IOL fixation method has proven t o be a simple method w ith good results in postoperative visual acuity, short operation time, and less astigmatism without serious corneal endothelium decompensation [
7,
11]. Ciliary sulcus IOL implantation induces a myopic shift when IOL power, which is calculated for the in-the-bag implantation, is used [
16-
19]. However, to the best of our knowledge, there have been no reports of postoperative refractive outcomes in the case of intrascleral fixation 2.5 mm posterior to the limbus. Thus, this study aimed to compare the clinical and refractive outcomes of intrascleral fixation of three-piece IOLs 2.5 mm posterior to the limbus with ciliary sulcus implantation and transscleral fixation 2.5 mm posterior to the limbus.
Materials and Methods
Ethical statements
The Institutional Review Board of Korea University Ansan Hospital approved this retrospective study (No. 2022AS0156). All research and data collection procedures adhered to the tenets of the Declaration of Helsinki.
Study population
This study retrospectively reviewed the medical records of the patients who underwent ciliary sulcus implantation, transscleral fixation, and intrascleral fixation with anterior vitrectomy at Korea University Ansan Hospital, between October 1, 2014, and July 31, 2021. Patients who had prior corneal refractive surgery or keratoplasty, who underwent complete pars plana vitrectomy, and who had postoperative complications were excluded. Cases with capture of the sulcus-implanted IOL optic by capsular opening were also excluded. Only cases with AMO Sensar AR40e (Johnson & Johnson Vision), a foldable three-piece IOL, were included to eliminate potential clinical differences arising from different types of IOLs.
Patient examination
All patients received preoperative comprehensive ocular examinations using slit-lamp biomicroscopy, fundus exam, and IOLMaster 500 (Carl Zeiss Meditec). IOL power was calculated using the Sanders-Retzlaff-Kraff/Theoretical (SRK/T) formula. In sulcus implantation, the IOL with adjusted power was used [
16,
18-
20]. In scleral fixation, the IOL power for the in-the-bag implantation was used since a previous study showed a small amount of myopic shift when IOL scleral fixation was done 2.5 mm posterior to the limbus [
2,
21]. In order to compare the postoperative refractive prediction error (diopters [D]) between the surgical methods, the predicted refraction was calculated as the in-the-bag implantation method to calculate the postoperative refractive prediction error. The A constant was 118.7 for AR40e IOL.
Surgical techniques
All surgical procedures were performed by one experienced surgeon (YE) under topical anesthesia with 0.5% proparacaine hydrochloride. Anterior vitrectomy was performed (Stellaris PC, Bausch & Lomb) to remove prolapsed vitreous from the anterior chamber, when needed. In addition to topical anesthesia, pinpoint anesthesia with 2% lidocaine was adopted in cases of scleral fixation [
22]. All patients went through one of the following surgical procedures.
1) Ciliary sulcus implantation
Sulcus implantation was performed when the capsular bag was compromised, including circumstances such as posterior capsular rupture, that prevent the IOL from being implanted in the bag. The IOL was loaded into the Emerald XL series cartilage (Johnson & Johnson Vision) and carefully injected into the sulcus.
2) Modified J transscleral fixation technique without tying up haptics
After pinpoint anesthesia with 2% lidocaine, four parallel points were marked at 2.5 mm posterior to the limbus and 6 mm vertically apart at approximately 2, 4, 8, and 10 oŌĆÖclock positions, and conjunctival flaps were made at each point [
9]. Double-armed 9-0 polypropylene straight needles were used. One straight needle was inserted 2.5 mm posterior to the limbus at the 8 oŌĆÖclock position and externalized 2.5 mm posterior to the limbus through a 26-guage needle at the 4 oŌĆÖclock position using the
ab externo approach. Polypropylene monofilament between 4 and 8 oŌĆÖclock positions was pulled out of the eye through a corneal or scleral incision using a Kuglen hook and wound twice over the leading haptic of a three-piece IOL. Then, the IOL was inserted into the anterior chamber with the trailing haptic kept outside the eye. The straight needle at the 4 oŌĆÖclock position was moved to the 2 oŌĆÖclock position through the sub-TenonŌĆÖs space and scleral tunnel. Then, the straight needle was inserted 2.5 mm posterior to the limbus at the 2 oŌĆÖclock position and externalized 2.5 mm posterior to the limbus through a 26-guage needle at the 10 oŌĆÖclock position using the
ab externo approach. Monofilament between 2 and 10 oŌĆÖclock positions was pulled out of the eye and wound twice over the trailing haptic of a three-piece IOL. The trailing haptic was inserted into the anterior chamber, and the IOL was centrally located in the posterior chamber. After the other straight needle at the 8 oŌĆÖclock position was moved to 10 oŌĆÖclock through the sub-TenonŌĆÖs space and scleral tunnel, both ends of polypropylene were tied at the 10 oŌĆÖclock position and the suture was buried in the scleral tunnel using a modified Baykara technique without a scleral flap [
23]. The conjunctiva was approximated using a 10-0 nylon suture.
3) Modified Yamane intrascleral haptic fixation technique
After pinpoint anesthesia with 2% lidocaine, two incision sites were marked at 2.5 mm posterior to the limbus at 5 and 11 oŌĆÖ clock positions on the sclera. A three-piece IOL was inserted into the anterior chamber through a corneal or scleral incision with the trailing haptic kept outside the eye. A 26-gauge needle was used to penetrate the marked scleral site at the 11 oŌĆÖclock position, and the leading haptic was subsequently fixed into the lumen of the needle using forceps and externalized onto the sclera. The end of the leading haptic was melted to form a flange using a high-temperature cautery probe. Another angled sclerotomy was performed with a 26-gauge needle at the 5 oŌĆÖclock position, and the same procedure was repeated to externalize the trailing haptic, which was melted to form a flange using a high-temperature cautery probe. The flange was then pushed back into the scleral tunnel to be fixed firmly [
2].
Preoperative and postoperative medication
For postoperative management, all patients began to use 0.5% or 1.5% levofloxacin hydrate (Cravit, Santen) and 1% prednisolone acetate (Pred-forte, Allergan Pharmaceutical) every 2 hours after the surgery. Beginning on the day after the surgery, the two eye drops were applied every 6 hours for the following month. Corneal and conjunctival sutures were removed 1 week after the surgery.
Patient evaluation
Postoperative corrected distance visual acuity (CDVA) at 4 m in the logarithm of the minimum angle of resolution (logMAR) and manifest refraction were measured at postoperative visits between 4 and 10 weeks. The refractive prediction error was defined as the difference between the preoperatively predicted refraction for the in-the-bag implantation using the SRK/T formula and the postoperative manifest refractive spherical equivalent observed 4 to 10 weeks after surgery. The back-calculated effective lens position (ELP) was defined as the postoperatively calculated ELP based on preoperative keratometry, axial length, IOL power, and postoperative refraction [
24].
Statistical analyses
Statistical analysis was performed using IBM SPSS ver. 20.0 (IBM Corp). A Kruskal-Wallis test and chi-square test were performed to compare the baseline demographic characteristics, postoperative refractive prediction error, back-calculated ELP, CDVA, cylinder power, and the proportion of patients with CDVA of 0.1 logMAR (Snellen, 20 / 25) or better among the ciliary sulcus implantation, transscleral fixation, and intrascleral fixation groups. A Mann-Whitney U-test and chi-square test were performed to investigate the effect of laterality on the postoperative refractive prediction error, back-calculated ELP, CDVA, cylinder power, and the proportion of patients with CDVA of 0.1 logMAR or better. Data are presented as median (interquartile range [IQR]), except for sex and laterality, which are in number (percentage). A p-value of <0.05 were considered statistically significant.
Results
A total of 65 eyes of 65 patients who underwent ciliary sulcus implantation, transscleral fixation, or intrascleral fixation 2.5 mm posterior to the limbus with the use of AMO Sensar AR40e IOL were included in the study. There were 33 patients with ciliary sulcus implantation, 10 patients with transscleral fixation using a modified J technique, and 22 patients with intrascleral fixation using a modified Yamane technique.
Table 1 compares the baseline demographic characteristics among the three groups. Except for laterality, there was no significant difference in age, sex, keratometry, anterior chamber depth, axial length, and IOL power among the three groups.
The causes of out-of-the-bag IOL implantation are listed in
Table 2: 22 eyes (33.8%) with posterior capsule rupture, 15 eyes (23.1%) with dislocated IOLs, 14 eyes (21.5%) with severe zonulysis, nine aphakic eyes (13.8%), three eyes (4.6%) with radial tear, and two eyes (3.1%) with calcified IOLs. The most common cause of ciliary sulcus IOL implantation was posterior capsular rupture (20 eyes, 60.6%), followed by IOL dislocation (five eyes, 15.2%). In contrast, severe zonulysis was the most common cause of IOL transscleral fixation (seven eyes, 70.0%), and dislocated IOL was the most common cause of IOL intrascleral fixation (nine eyes, 40.9%). In the cases of IOL dislocation through posterior capsule opening, the IOL was inserted into the ciliary sulcus when the most of the CCC was intact.
There were two incision types for the surgical approaches. The surgical approaches could be implemented by using either a clear corneal incision or a scleral tunnel incision. While clear corneal incision approaches were adopted for all ciliary sulcus implantation cases, there were 21 cases of clear corneal incision and 11 cases of scleral tunnel incision in the scleral fixation. All 11 cases of scleral tunnel incision were those who underwent intracapsular cataract extraction in eyes with severe zonulysis (
Table 3).
As there was a significant difference in laterality among the three groups, this study performed a Mann-Whitney U-test and chi-square test to investigate the effect of laterality on the postoperative refractive prediction error, back-calculated ELP, CDVA, cylinder power, and the proportion of patients with CDVA of 0.1 logMAR or better. There were no significant differences in the postoperative refractive prediction error (right eye, ŌłÆ0.31 D [IQR, ŌłÆ0.81 to ŌłÆ 0.02 D]; left eye, ŌłÆ 0.71 D [IQR, ŌłÆ1.07 to ŌłÆ 0.22 D]; p = 0.122), the back-calculated ELP (right eye, 4.56 mm [IQR, 4.39 to 4.92 mm]; left eye, 4.41 mm [IQR, 4.10 to 4.84 mm]; p = 0.133), CDVA (right eye, 0 logMAR [IQR, 0 to 0.10 logMAR]; left eye, 0 logMAR [IQR, 0 to 0.10 logMAR]; p = 0.898), cylinder power (right eye, ŌłÆ0.88 D [IQR, ŌłÆ1.81 to ŌłÆ0.50 D]; left eye, ŌłÆ0.75 D [IQR, ŌłÆ1.50 to ŌłÆ0.25 D]; p = 0.371), and the proportion of patients with CDVA of 0.1 logMAR or better (right eye, 80%; left eye, 80%; p > 0.999) between the right and left eyes.
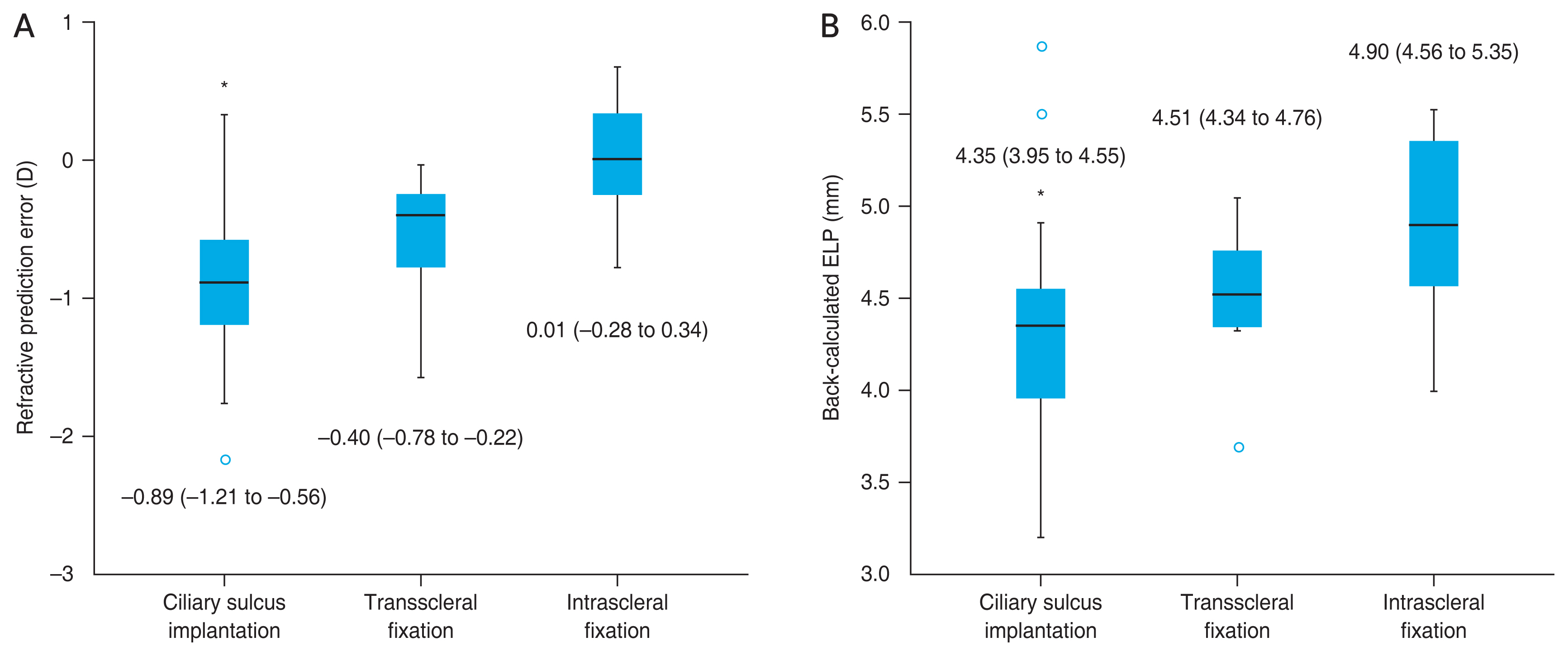
The postoperative refractive prediction error in the ciliary sulcus implantation group (ŌłÆ0.89 D; IQR, ŌłÆ1.21 to ŌłÆ0.56 D) showed a greater myopic shift than that in the transscleral fixation (ŌłÆ0.40 D; IQR, ŌłÆ0.78 to ŌłÆ0.22 D) and intrascleral fixation groups (0.01 D; IQR, ŌłÆ0.28 to 0.34 D; all
p < 0.001) (
Fig. 1A). The back-calculated ELP of the ciliary sulcus implantation group (4.35 mm; IQR, 3.95 to 4.55 mm) was significantly smaller than that of the transscleral fixation (4.51 mm; IQR, 4.34 to 4.76 mm) and intrascleral fixation groups (4.90 mm; IQR, 4.56 to 5.35 mm; all
p < 0.001) (
Fig. 1B). When the IOL is located through transscleral fixation by winding thread on the optic-haptic junction, it theoretically becomes more anteriorly located than when it is fixated at the tip of the haptic via intrascleral fixation due to the positional difference that results from the haptic angle of 5┬░ (
Fig. 2).
When the ciliary sulcus implantation, transscleral fixation, and intrascleral fixation groups were compared, there were no significant differences in the postoperative CDVA at 4 months (0 logMAR [IQR, 0 to 0.05 logMAR], 0.10 logMAR [IQR, 0 to 0.30 logMAR], and 0 logMAR [0 to 0.10 logMAR], respectively; p = 0.083) and residual cylinder power (ŌłÆ0.75 D [IQR, ŌłÆ1.50 to ŌłÆ 0.50 D], ŌłÆ1.13 D [IQR, ŌłÆ2.06 to ŌłÆ0.75 D], and ŌłÆ0.63 D [IQR, ŌłÆ1.13 to ŌłÆ0.25 D], respectively; p = 0.074). The proportion of patients with CDVA of 0.1 logMAR (Snellen, 20 / 25) or better was 82%, 60%, and 86% in the ciliary sulcus implantation, transscleral fixation, and intrascleral fixation groups, respectively (p = 0.218).
Discussion
This study compared the clinical outcomes of three different IOL insertion methods using a single type of three-piece IOL: ciliary sulcus implantation, transscleral fixation, and intrascleral fixation. The results showed significantly different values of refractive prediction error and ELP among the three groups. The analysis of the postoperative refractive prediction error showed that the ciliary sulcus implantation group had the greatest myopic shift, whereas the intrascleral fixation group had the least myopic shift, closer to the emmetropic target refraction. In addition, the back-calculated ELP was the lowest with ciliary sulcus implantation and greatest with intrascleral fixation. This implies that the IOL within the ciliary sulcus is most anteriorly located; the IOL with transscleral fixationŌĆöespecially with the J techniqueŌĆöis in the middle; and the IOL with intrascleral fixationŌĆöwith the Yamane techniqueŌĆöis the most posteriorly located.
A myopic shift from the target refraction is anticipated when the IOL is anteriorly located relative to the expected in-the-bag location [
17-
19]. Indeed, when the IOL is implanted in the ciliary sulcus, its position is expected to be approximately 0.5 mm more anterior than its in-the-bag lens position [
25]. Jang and Lee [
26] retrospectively reviewed 19 patients who underwent ciliary sulcus implantation and found a refractive deviation to be ŌłÆ0.71 ┬▒ 1.02 D. Suto et al. [
16] reported a refractive deviation of ŌłÆ0.78 ┬▒ 0.47 D in the ciliary sulcus implantation group consisting of 30 patients. Choi et al. [
17] reported a refractive deviation of ŌłÆ0.93 ┬▒ 0.68 D after IOL implantation in the ciliary sulcus. In the current study, the median refractive deviation for ciliary sulcus implantation was ŌłÆ0.89 D, which is consistent with the findings from the previous studies [
16,
17,
26].
The degree and order of myopic shift of the three surgical methods clinically correspond with their lens positions. All surgeries in this study used AMO Sensar AR40e IOL, which has a 6-mm optic diameter and two haptics made of polymethyl methacrylate with a 5┬░ haptic angulation. Postoperative refraction changes are known to be influenced by types and designs of IOLs used [
27]. Potential refractive differences due to different types of IOLs can be eliminated by using a single IOL type. Compared to transscleral fixation with winding thread at the optic-haptic junction, intrascleral fixation seemed to induce a more emmetropic target and ELP closer to the expected capsular in-the-bag position because it is fixated at the tip of the haptic (
Fig. 2).
In this study, all IOLs in the scleral fixation group were placed at 2.5 mm posterior to the limbus. The original Yamane technique involves flanged fixation at 2.0 mm from the limbus. Scleral fixation is usually done between 2.0 and 2.5 mm from the limbus, because IOL fixation of less than 2.0 mm may lead to damage in the iris and ciliary body and consequent anterior chamber inflammation [
10]. It is important to locate the IOL in the appropriate anteroposterior position, where haptics are placed posterior to the ciliary body processes without touching, but simultaneously anterior to the ora serrata. If the IOL is fixated too anteriorly, its contact with the ciliary body processes can ultimately lead to chronic inflammation and bleeding. However, if the IOL is fixated too posteriorly, it may result in retinal breaks, hemorrhages, or even detachments [
28]. The distance between the end of the pars plicata containing the ciliary processes and the posterior surgical limbus is known to be approximately 2.41 mm. This position is considered to be a critical boundary because needle insertion anterior to 2.41 mm may penetrate the ciliary body processes or cause bleeding of the pars plicata [
29]. Therefore, it appears that fixation 2.5 mm posterior to the limbus is anatomically safe with good postoperative refractive outcomes.
All cases of intrascleral haptic fixation technique in this study used a 26-gauge needle to penetrate the sclera. In 2014, Yamane et al. [
6] first introduced a sutureless intrascleral fixation method with a 27-gauge needle to externalize the haptics. Although this 27-gauge needle-guided fixation proved to be a feasible technique with good IOL fixation without any wound leak, there was a potential risk of IOL tilt or dislocation. Yamane et al. [
7] then devised a new flanged IOL fixation method using 30-gauge needles, which was a simple and less invasive method with good IOL fixation. Compared to the 27-gauge needle technique, sutureless IOL flanged fixation using a 30-gauge needle demonstrated greater IOL stability and centration with less postoperative complications [
30]. In this study, although intrascleral fixation was conducted using a 26-gauge needle- guided technique, there were no cases of IOL dislocation or decentration during the follow-up period.
There are several limitations in this study. The study was retrospective in nature. The sample size was small, and the patients were not randomly assigned between the two fixation groups. The surgeon chose one of the two fixation methods in chronological order, with the transscleral fixation technique being used until 2018 and the intrascleral haptic fixation technique being used after 2018. The two techniques resulted in different ratios of laterality among the groups, even though the surgical method and surgical outcome were unaffected by which eye (right or left) was being operated on. The follow-up periods were not long enough to evaluate long-term clinical outcomes and potential late complications such as IOL dislocation. As all the surgeries were done by a single surgeon, the result of this study may not apply to other surgeons. Other surgical outcomes such as surgical time and intraoperative and postoperative complications were not included in the study. Thus, further studies would be needed to address additional findings.
In conclusion, this study compared the clinical outcomes, including postoperative refraction, IOL position, visual acuity, and astigmatism, in patients who underwent ciliary sulcus implantation, transscleral fixation, or intrascleral fixation. There were significant differences in the postoperative refractive prediction error and IOL position among the different surgical methods. The IOLs were the most posteriorly located and were closer to the emmetropic target refraction with intrascleral fixation 2.5 mm posterior to the limbus. Due to the haptic-angulated nature of AMO Sensar AR40e, the IOLs implanted by intrascleral fixation with the modified Yamane technique were more posteriorly located than the IOLs with transscleral fixation with winding thread at the optic-haptic junction. However, there was no significant difference in postoperative visual acuity or residual astigmatism, implying that all three surgical procedures are effective with respect to IOL stability and good vision outcome.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




