 |
 |
| Korean J Ophthalmol > Volume 37(1); 2023 > Article |
|
Abstract
Purpose
Human corneal endothelial progenitor cells (HCEPs), which has been selectively isolated and differentiated into human corneal endothelial cells (HCECs), are crucial for repairing corneal endothelial damage. In this study, we evaluated the roles of a Rho-assisted kinase (ROCK) inhibitor, Y-27632, on the isolation and expansion of HCEPs, and assessed the in vitro effects of different concentrations of Y-27632 on the differentiated HCEPs.
Methods
HCEPs were isolated and expanded in a medium with and without 10μM Y-27632, and then differentiated into HCECs in a medium with fetal bovine serum. The characteristics of HCEPs and differentiated HCEPs were confirmed by immunofluorescence staining. The proliferation, viability, morphology, and wound-healing ability of differentiated HCEPs were assessed in the presence of different concentrations of Y-27632.
Results
Y-27632 enabled the isolation and expansion of HCEPs from the corneal endothelium. The differentiated HCEPs showed an optimal increase in proliferation and survival in the presence of 10μM Y-27632. As the concentration of Y-27632 increased, differentiated HCEPs became elongated, and actin filaments were redistributed to the periphery of cells. Y-27632 also caused a concentration-dependent enhancement in the wound-healing ability of differentiated HCEPs.
Human corneal endothelial cells (HCECs) have limited proliferative potential in vivo [1,2]; therefore, damage to CECs leads to irreversible endothelial dysfunction and corneal edema. The conventional treatment of severe corneal endothelial dysfunction is corneal transplant, which consists of either the entire corneal layer or only the posterior lamellar corneal layer, derived from donated corneal tissue. To overcome the shortage of donor corneas, transplantation of cultivated HCECs has been suggested as an alternative treatment [3-9]. Many groups have successfully cultured and amplified HCECs in vitro, and treated animal models with corneal endothelial dysfunction [10,11]. However, there are no defined protocols for the clinical application of cultured HCECs. In addition, current corneal endothelial engineering has restriction, such as limited proliferative ability, fibroblastic transformation, and cellular senescence [12-18].
Several studies have reported the presence of human corneal endothelial progenitor cells (HCEPs) [19-23]. Hara et al. [24] had established a method for culturing HCEPs and differentiating them into HCECs. They had suggested that, unlike conventional HCEC culture, HCEPs could be selectively expanded with high proliferative potency and to generate transplantable CEC sheets [24]. Using progenitor cells in this manner could help overcome the limitations of corneal endothelial engineering.
Recently, cultured CECs were injected into the anterior chamber of the eye along with an inhibitor of Rho-associated kinase (ROCK) [18]. ROCK inhibition enhanced cell engraftment at the posterior corneal layer, enabling the cell-based treatment of corneal endothelial dysfunction [18,25]. This was consistent with many reports that demonstrated that the inhibition of ROCK signaling decreased apoptosis and increased proliferation and cellular adhesion in the CECs cultured by various methods [12,18,26].
The aim of this study was to evaluate the role of the ROCK inhibitor, Y-27632, on the isolation and expansion of HCEPs, and to assess the in vitro effects of different concentrations of Y-27632 on the differentiated HCEPs. The results of the study might contribute to cell injection treatments of differentiated HCEPs, for corneal endothelial dysfunction.
The study protocol was approved by the Institutional Review Board of Inha University Hospital (No. 2016-05-018) and adhered to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from the next of kin of all deceased donors. Ten human peripheral corneal tissues from eight donors (mean donor age, 54.6 ± 19.4 years) were obtained by trephination with 7.0-mm trephines and stored in storage medium (OptiSol-GS, Bausch & Lomb) at 4°C.
HCEPs were isolated and cultured as previously described by Hara et al. [24]. Briefly, Descemet’s membranes with HCECs were stripped from the human corneas using sterile surgical forceps. The tissue was transferred to an enzyme cell detachment medium (Accutase, Life Technologies) at 37°C for 30 minutes, and centrifuged at 15,000 rpm for 5 minutes. The cells were seeded at a density of 100 to 300 cells/cm2 onto culture plates coated with 20 μg/mL laminin-511 (BioLamina).
The culture medium comprised Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12), supplemented with 20% knockout serum replacement (KSR), 2mM L-glutamine, 1% nonessential amino acids, 100μM 2-mercaptoethanol, 50 U/mL penicillin G, and 50 μg/mL streptomycin (all from Life Technologies) along with 4 ng/mL basic fibroblast growth factor (bFGF) and 10μM Y-27632 (both from Wako Pure Chemical Industrials). Paired corneas from two donors were used to compare the expansion of HCEPs with and without 10μM Y-27632. HCEPs were cultured in a humidified atmosphere with 5% CO2 at 37°C, and the culture medium was changed every 2 to 3 days. When the cells reached confluence, they were harvested with Accutase and passaged at ratios of 1:2 to 1:4.
HCEPs were differentiated into HCECs on culture plates coated with fibronectin, collagen, and albumin coating mix (AthenaES). The differentiation medium was composed of low-glucose DMEM with 10% fetal bovine serum, 50 U/mL penicillin G, and 50 μg/mL streptomycin (all from Life Technologies). The differentiated HCEPs were cultured in a humidified atmosphere with 5% CO2 at 37°C. When the cells reached 70% confluency, they were harvested with 0.05% Trypsin-EDTA (Life Technologies) and passaged at a ratio of 1:4. The differentiated cells were viewed using optical microscope (Olympus CKX41, Olympus).
The HCEPs and differentiated HCEPs were cultured on 8-well culture slides (BD Biosciences) for immunofluorescence staining. They were fixed with 80% acetone at −20°C for 10 minutes, and nonspecific absorption was blocked with an antibody diluent solution (Life Technologies) at 37°C for 20 minutes. The cells were incubated overnight at 4°C with the primary antibodies against p75 neurotrophin receptor (p75NTR; 1:100, Merck Millipore), SOX9 (1:100, Abcam), ZO-1 (1:100, Life Technologies), and Na+/K+-ATPase (1:100, Merck Millipore) in an antibody diluent solution. They were washed three times in phosphate-buffer saline with 0.1% Tween 20 (PBS-T). The cells were then incubated for 2 hours in a 1:200 dilution of rhodamine-labeled goat anti-mouse immunoglobulin G and human serum absorbed fluorescein labeled goat anti-rabbit immunoglobulin G (both from KPL), and again washed three times in PBS-T in the dark. To stain the nuclei, the cells were stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Thermo) for 5 minutes. After mounting, the cells were observed using a fluorescence microscope (Olympus BX43, Olympus), and images were processed using the ISCapture Professional Imaging Software (Tucsen).
Radioimmunoprecipitation assay buffer (50mM Tris-HCl, 100mM NaCl, 5mM EDTA, 1% sodium deoxycholate, 4% Triton X-100, 0.4% sodium dodecyl sulfate) containing a protease and phosphatase inhibitor mini tablets (Thermo Fisher Scientific) was used for isolation of total cellular proteins. Western blotting was conducted using standard protocols. The 5% bovine serum albumin (Amresco) in Tris (hydroxymethyl) aminomethane-buffered saline (pH 7.4) with 0.01% Tween 20 (TBS-T) was used for blocking the nonspecific binding for 1 hour. Primary antibodies were ZO-1/TJP1 antibody (Thermo Fisher Scientific, 1:200 dilution), Na+/K+-ATPase antibody (Merck Millipore, 1:200 dilution). A horseradish peroxidase conjugated secondary antibody with a ECL Prime Western blotting detection reagent (GE Healthcare) was used for detection of immunoreactive bands.
The differentiated HCEPs were cultured on 8-well culture slides (BD Biosciences) with and without 10μM Y-27632 for 24 hours and fixed and incubated overnight at 4°C with purified mouse anti-Ki67 antibodies (1:100, BD Biosciences). The cells were then incubated with goat anti-mouse secondary antibodies for 2 hours as described in the previous section and washed three times in PBS-T in the dark. The nuclei were stained with DAPI, and cells were visualized as previously described.
The differentiated HCEPs were plated at a density of 2,000 cells per well on 96-well plates in a medium containing 0μM, 5μM, 10μM, and 30μM Y-27632 for 24 hours. The number of viable differentiated HCEPs was determined using the cell counting kit-8 (Dojindo Molecular Technologies Inc). Absorbance was measured at 450 nm to determine cell viability in each well using a Universal Microplate Reader ELx800G (BioTek Instruments Inc).
The differentiated HCEPs were stained with immunofluorescent actin to evaluate their morphologic changes when treated with the different concentrations of Y-27632. The cells were cultured on 8-well culture slides with 0μM, 5μM, 10μM, and 30μM Y-27632 for 24 hours. The cells were fixed and incubated overnight at 4°C in 1:200 dilution Alexa Fluor 594-conjugated phalloidin (Life Technologies), and washed three times with PBS-T. After counterstaining with DAPI, the cells were visualized in a fluorescence microscope as described in previous sections.
The differentiated HCEPs were cultured until confluence in 60-mm culture dishes and scraped with a 200-μL plastic pipette tip. The unattached cells were washed away in PBS before the remaining cells were incubated in media with 0μM, 5μM, 10μM, and 30μM Y-27632. The extent of the wound was determined by estimating the area between cells at the two opposite edges of the defect. Images of migrating of cells were obtained using an optical microscope, and relative wound sizes were analyzed using the ImageJ software (US National Institutes of Health) after 0, 6, 24, and 48 hours of incubation.
All the data are represented as mean ± standard deviation. Statistical significance was determined using Student t-test for single comparison and one-way analysis of variance, followed by Tukey’s test for multiple comparisons. A p-value of <0.05 was considered statistically significant.
A comparative study was performed on paired corneas from two donors (48 and 46 years old) to evaluate the role of Y-27632 in the isolation and expansion of HCEPs. When 10μM Y-27632 was added, HCEPs from both donors were successfully isolated and expanded on a laminin-511-coated dish, while HCEPs were not observed in the absence of Y-27632, even after 30 days of culture (Fig. 1A, 1B). The cells had a bipolar, spindle-shaped morphology, and expressed the neural crest markers, p75NTR and SOX9, which are known markers of HCEPs [24]. HCEPs from the corneas of eight donors were subcultured for several passages (mean numbers of passages, 4.8 ± 1.8) (Fig. 2A, 2B).
We confirmed that HCEPs in low passages (below three passages) were differentiated into HCECs in the differentiation medium. The morphology of differentiated HCEPs changed from bipolar and spindle-shaped to confluent and hexagonal through the passages (mean numbers of passages, 7.8 ± 2.1) (Fig. 3A). In addition, the differentiated HCEPs displayed characteristic expression of ZO-1 and Na+/K+-ATPase in the plasma membrane (Fig. 3B) and this confirmed by Western blotting (Fig. 3C).
We used the differentiated HCEPs from passages 2 to 7, which confirmed the expression of ZO-1 and Na+/K+-ATPase.
To determine the effects of Y-27632 on the proliferation of differentiated HCEPs, we used Ki67 immunostaining as a reliable marker of cell proliferation. The differentiated HCEPs treated with 10μM Y-27632 had significantly more Ki67 positive cells than the untreated control cells (p = 0.028) (Fig. 4A, 4B).
To determine the optimal concentration of Y-27632 required for obtaining viable differentiated HCEPs, we performed a cell viability assay after 24-hour exposure to different concentrations of Y-27632. The number of adherent and viable differentiated HCEPs increased as the Y-27632 concentration increased from 0μm to 10μm, then decreased at a 30μm concentration (p = 0.003). At 10μm, viability was significantly higher than that in the untreated control (p = 0.006) (Fig. 5).
The effect of Y-27632 on the morphology of differentiated HCEPs was examined by immunostaining with phalloidin, which is used to assess the distribution pattern of actin filaments. As the concentration of Y-27632 increased, the differentiated HCEPs lost their polygonal shape and became more elongated. The differentiated HCEPs remained polygonal at concentrations of Y-27632 below 10μm. With 30μm Y-27632, a large proportion of differentiated HCEPs showed the elongated morphology. The actin filaments were also altered, changing from a central radial distribution into a peripheral distribution along the plasma membrane (Fig. 6).
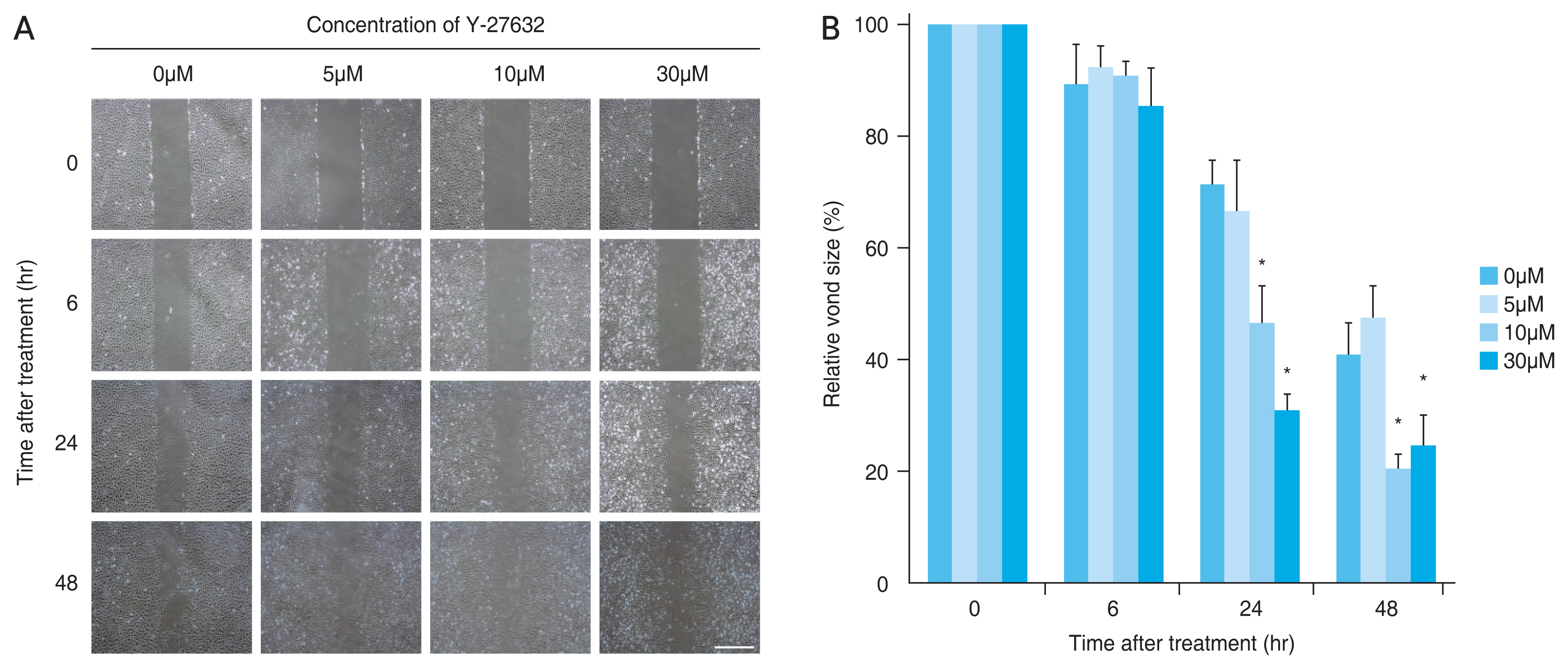
We observed a concentration-dependent enhancement in the wound-healing ability of differentiated HCEPs treated with Y-27632. In response to the wound, differentiated HCEPs became elongated and fibroblast-like in appearance, and began to migrate from the scraping edge. At both 24 and 48 hours, after treatment with 10μm or 30μm Y-27632, statistically significant increases in wound closure rate, were observed, compared to the untreated control (at 24 hours, p = 0.005 and p < 0.001; at 48 hours, p = 0.005 and p = 0.018) (Fig. 7A, 7B).
Many studies have used various culture methods and supplements for transplanting cultured CECs, as an alternative treatment for corneal endothelial dysfunction [3-11]. However, there are several limitations to this method, and no defined protocols have been established for clinical application. Many groups have tried to resolve the current limitations in conventional HCEC amplification [10,11], and the use of corneal endothelial progenitor cells has also been explored [24]. HCEPs have been suggested to retain the proliferative capacity at the peripheral cornea [19-23,27].
In this study, we isolated and expanded HCEPs from peripheral corneas using KSR-based serum-free media supplemented with bFGF and laminin-511 [24], which have been used to maintain undifferentiated cells, such as human embryonic stem cells and mesenchymal stem cells [24,28,29]. Laminin-511 was considered important for isolating and expanding HCEPs, because the substrate has been used in the serum-free culture of human embryonic stem cells, and is present in the corneal Descemet’s membrane [24,30,31].
We cultured HCEPs using a protocol previously described by Hara et al. [24] who had briefly treated the stripped Descemet’s membranes with Y-27632 at the initial stage only, not throughout the culture period. Furthermore, they did not study the effects of Y-27632 on the culture method. In our study, we demonstrated that Y-27632 was necessary for the isolation and expansion of HCEPs. This finding was consistent with the results of many previous reports that demonstrated a significant increase in the adhesion of HCECs cultured with Y-27632 [12,32]. In one study, the addition of Y-27632 into a dual media culture system resulted in a twofold to threefold higher yield of CECs [33]. ROCK signaling is known to be involved in cell adhesion, morphogenesis, migration, and cell cycle progression [34]. A selective ROCK inhibitor, Y-27632, which is associated with increased adhesion and enhancement of actomyosin contractility, has been used for the in vitro culture of CECs in corneal endothelial regenerative medicine for several years [12,32-34].
Our results showed that bipolar, spindle-shaped HCEPs expressed p75NTR and SOX9, which are markers of neural crest cells [35,36]. Hara et al. [24] had reported that HCEPs had partially retained the properties of the neural crest and periocular mesenchyme and showed high expression of p75NTR, SOX9, and FOXC2. Another study had reported that bovine corneal endothelial precursor cells, isolated with a sphere-forming assay, expressed the neural crest stem cell marker, nestin, and that these cells had the potential to differentiate into CECs [19,24]. In our study, HCEPs also differentiated into hexagonal HCECs in a medium with fetal bovine serum. Thus, cultivating HCECs from isolated HCEPs with high proliferative potency could be a novel source of cells for treating corneal endothelial dysfunction, as demonstrated in the results of our study, as well as those of previous reports [19,24].
In a recent study, cultivated CECs with Y-27632, were directly injected into the anterior chamber of the eye, and this turned out to be a significant success in corneal regenerative medicine [18]. The ROCK inhibitor, Y-27632, increased the adhesion of injected cells onto the recipient cornea without any substrate, and thus helped in treating corneal endothelial dysfunction in animal models [12,18,34]. Therefore, as a first step towards the use of HCECs cultivated by isolating HCEPs for cell injection treatments, we evaluated the in vitro effects of Y-27632 on these HCECs, with the aim of determining the optimal concentration of Y-27632.
Consisting with previous findings [12,26,34,37,38], our results showed that ROCK inhibition with 10μM Y-27632 promoted the proliferation and survival of HCECs. ROCK inhibitors are thought to control the expression of cyclin D and p27 via PI 3-kinase signaling, thus promoting CEC proliferation [26]. Previous studies have revealed the role of ROCK signaling in the regulation of proapoptotic and antiapoptotic, as well as cell survival signaling [39,40]. However, to confirm whether Y-27632 treatment was sufficient to induce HCECs proliferation, further research is needed as the other studies demonstrated conflicting results [32].
In this study, Y-27632 caused a concentration-dependent change in HCEC morphology; as the cells became elongated, and actin filaments were redistributed to the periphery. Y-27632 also enhanced wound healing via cell migration and morphologic changes in HCECs, which was consistent with the results of previous studies [41,42]. The cell migration involved membrane protrusion through cytoskeleton modification. Pipparelli et al. [32] had revealed that the inhibition of ROCK signaling resulted in morphological changes in HCECs, characterized by loss of their polygonal shape and a remodeling of the cytoskeleton, as demonstrated by the redistribution of actin to the cell periphery. They proposed that the mechanism behind the enhanced wound-healing effect of Y-27632 was associated with the regulation of the actin cytoskeleton.
Most studies have used a concentration around 10μM to 30μM of Y-27632 for the culture of CECs [32,33]; the optimal Y-27632 concentration is yet to be determined. With 30μM Y-27632, we observed a slight decrease in the cell survival and wound healing of HCECs, compared to those of the HCECs treated with 10μM Y-27632. Peh et al. [33] had shown a dose-dependent decrease in the attachment strength after the CECs were exposed to more than 30μM of Y-27632. Therefore, we suggested 10μM as the optimal concentration of Y-27632 for HCECs cultured by isolating HCEPs.
In conclusion, our results showed that the ROCK inhibitor, Y-27632, enabled the isolation and expansion of HCEPs from peripheral corneal tissue. We observed optimal proliferation and survival of differentiated HCEPs with 10μM Y-27632. The ROCK inhibitor also induced morphological changes and enhanced wound healing in differentiated HCEPs. Further studies are required to evaluate the therapeutic effects of injecting transplantable differentiated HCEPs with Y-27632 in vivo.
References
2. Joyce NC, Meklir B, Joyce SJ, Zieske JD. Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci 1996;37:645-55.

3. Mimura T, Yamagami S, Yokoo S, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci 2004;45:2992-7.


4. Ishino Y, Sano Y, Nakamura T, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci 2004;45:800-6.


5. Sumide T, Nishida K, Yamato M, et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J 2006;20:392-4.



6. Mimura T, Yamagami S, Yokoo S, et al. Sphere therapy for corneal endothelium deficiency in a rabbit model. Invest Ophthalmol Vis Sci 2005;46:3128-35.


7. Mimura T, Yokoo S, Araie M, et al. Treatment of rabbit bullous keratopathy with precursors derived from cultured human corneal endothelium. Invest Ophthalmol Vis Sci 2005;46:3637-44.


8. Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci 1977;16:597-613.

9. Koizumi N, Sakamoto Y, Okumura N, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci 2007;48:4519-26.


10. Peh GS, Beuerman RW, Colman A, et al. Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation 2011;91:811-9.


11. Mimura T, Yamagami S, Amano S. Corneal endothelial regeneration and tissue engineering. Prog Retin Eye Res 2013;35:1-17.


12. Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci 2009;50:3680-7.


13. Peh GS, Toh KP, Wu FY, et al. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS One 2011;6:e28310.



14. Shima N, Kimoto M, Yamaguchi M, Yamagami S. Increased proliferation and replicative lifespan of isolated human corneal endothelial cells with L-ascorbic acid 2-phosphate. Invest Ophthalmol Vis Sci 2011;52:8711-7.


15. Okumura N, Kay EP, Nakahara M, et al. Inhibition of TGF-β signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS One 2013;8:e58000.



16. Nakahara M, Okumura N, Kay EP, et al. Corneal endothelial expansion promoted by human bone marrow mesenchymal stem cell-derived conditioned medium. PLoS One 2013;8:e69009.



17. Okumura N, Kakutani K, Numata R, et al. Laminin-511 and -521 enable efficient in vitro expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci 2015;56:2933-42.


18. Okumura N, Sakamoto Y, Fujii K, et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci Rep 2016;6:26113.




19. Yokoo S, Yamagami S, Yanagi Y, et al. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest Ophthalmol Vis Sci 2005;46:1626-31.


20. Yamagami S, Yokoo S, Mimura T, et al. Distribution of precursors in human corneal stromal cells and endothelial cells. Ophthalmology 2007;114:433-9.


21. Whikehart DR, Parikh CH, Vaughn AV, et al. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis 2005;11:816-24.

22. McGowan SL, Edelhauser HF, Pfister RR, Whikehart DR. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol Vis 2007;13:1984-2000.

23. He Z, Campolmi N, Gain P, et al. Revisited microanatomy of the corneal endothelial periphery: new evidence for continuous centripetal migration of endothelial cells in humans. Stem Cells 2012;30:2523-34.



24. Hara S, Hayashi R, Soma T, et al. Identification and potential application of human corneal endothelial progenitor cells. Stem Cells Dev 2014;23:2190-201.


25. Koizumi N, Okumura N, Kinoshita S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp Eye Res 2012;95:60-7.


26. Okumura N, Nakano S, Kay EP, et al. Involvement of cyclin D and p27 in cell proliferation mediated by ROCK inhibitors Y-27632 and Y-39983 during corneal endothelium wound healing. Invest Ophthalmol Vis Sci 2014;55:318-29.


27. Hirata-Tominaga K, Nakamura T, Okumura N, et al. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cells 2013;31:1396-407.



28. Battula VL, Bareiss PM, Treml S, et al. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation 2007;75:279-91.


29. Schuldiner M, Yanuka O, Itskovitz-Eldor J, et al. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 2000;97:11307-12.



30. Rodin S, Domogatskaya A, Strom S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol 2010;28:611-5.



31. Kabosova A, Azar DT, Bannikov GA, et al. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci 2007;48:4989-99.



32. Pipparelli A, Arsenijevic Y, Thuret G, et al. ROCK inhibitor enhances adhesion and wound healing of human corneal endothelial cells. PLoS One 2013;8:e62095.



33. Peh GS, Adnan K, George BL, et al. The effects of Rho-associated kinase inhibitor Y-27632 on primary human corneal endothelial cells propagated using a dual media approach. Sci Rep 2015;5:9167.




34. Okumura N, Koizumi N, Ueno M, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol 2012;181:268-77.


35. Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 1999;96:737-49.


36. Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development 2003;130:5681-93.



37. Bi YL, Zhou Q, Du F, et al. Regulation of functional corneal endothelial cells isolated from sphere colonies by Rho-associated protein kinase inhibitor. Exp Ther Med 2013;5:433-7.



38. Li S, Wang C, Dai Y, et al. The stimulatory effect of ROCK inhibitor on bovine corneal endothelial cells. Tissue Cell 2013;45:387-96.


39. Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res 2000;261:44-51.


40. Riento K, Ridley AJ. ROCKs: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003;4:446-56.



Fig. 1
Isolation and expansion of human corneal endothelial progenitor cells (HCEPs) from the corneal tissue. (A) Phase-contrast images of Y-27632-treated HCEPs (scale bars, 300 μm) at days 1, 6, 13, and 15. (B) Phase-contrast image of cells cultured without Y-27632 (scale bars, 300 μm) at days 1, 6, 17, and 30.

Fig. 2
Characterization and expansion of human corneal endothelial progenitor cells (HCEPs). (A) Bipolar and spindle-shaped HCEPs from a 48-year-old donor proliferated during five passages (scale bars, 300 μm). (B) Expression of the HCEPs markers: p75 neurotrophin receptor (p75NTR; red) and SOX9 (green) in HCEPs (scale bars, 300 μm). DAPI = 4′,6-diamidino-2-phenylindole, dihydrochloride.

Fig. 3
Characterization and expansion of differentiated human corneal endothelial progenitor cells (HCEPs). (A) Polygonal differentiated HCEPs from a 22-year-old donor were proliferated for seven passages (scale bars, 300 μm). (B,C) Expression of the differentiated HCEPs functional markers ZO-1 and Na+/K+-ATPase (scale bars, 50 μm). DAPI = 4′,6-diamidino-2-phenylindole, dihydrochloride.
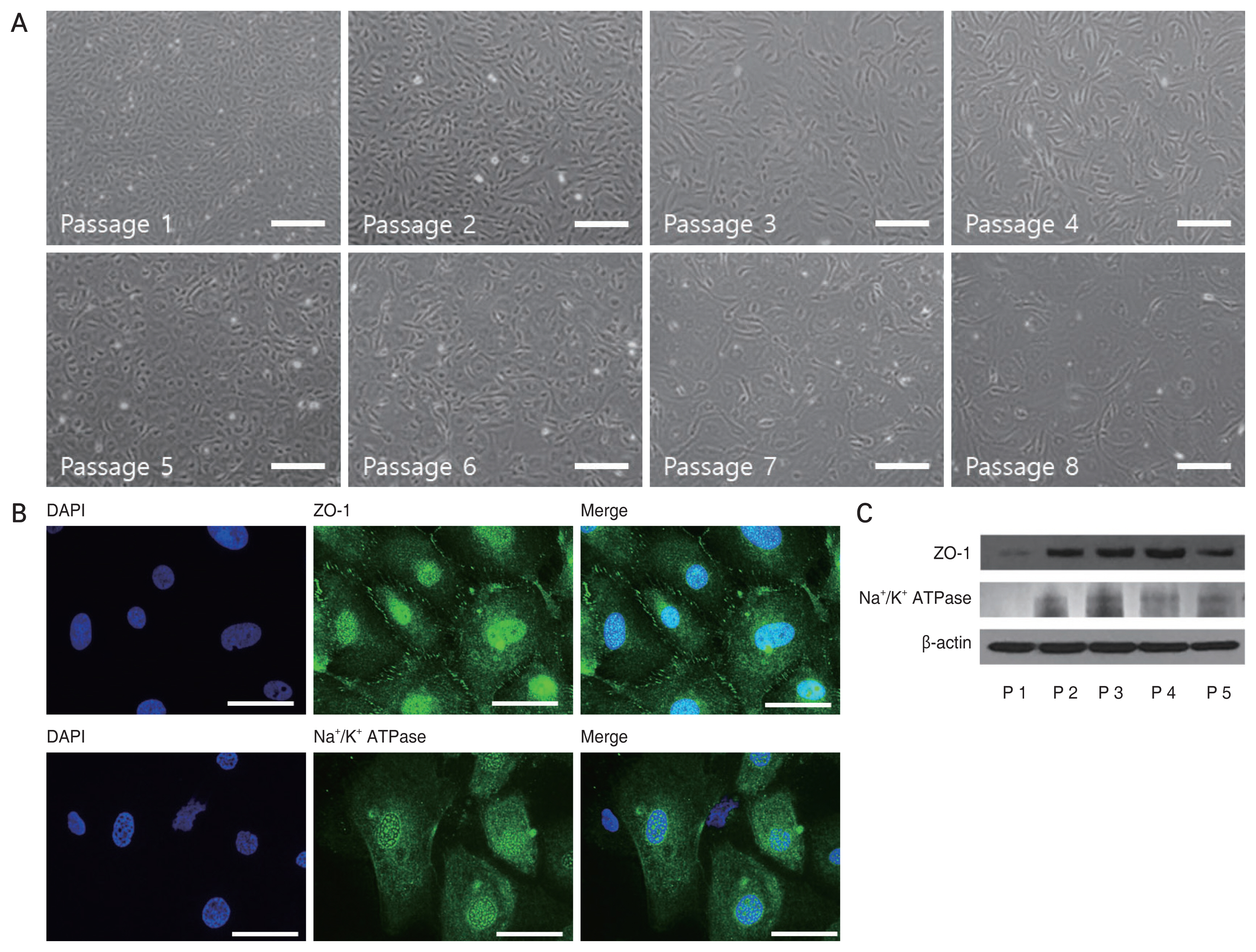
Fig. 4
Increased proliferation of differentiated human corneal endothelial progenitor cells (HCEPs) in the presence of 10μM Y-27632. (A) The differentiated HCEPs were immunostained for the proliferative marker, Ki67 (green), in the presence and absence of Y-27632. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; blue; scale bars, 300 μm). (B) The proliferative capacity of the cells was determined by calculating the ratio of Ki67-positive cells to total DAPI-stained cells (n = 5). *p < 0.05 (Student t-test).
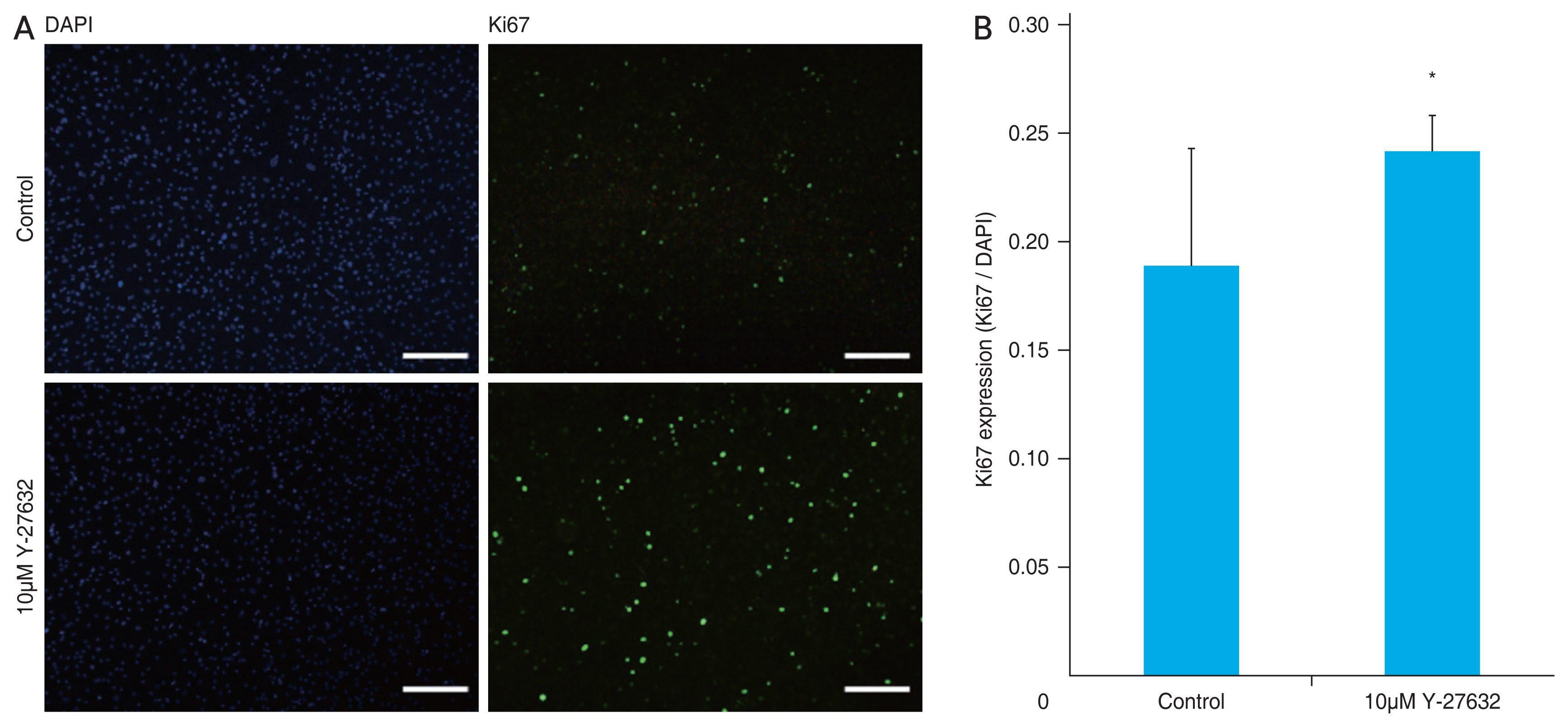
Fig. 5
Enhanced survival of differentiated human corneal endothelial progenitor cells (HCEPs) treated with Y-27632 treatment. The viability of differentiated HCEPs was evaluated using the cell counting kit-8 assay, in the presence of different concentrations of Y-27632 (n = 8). *p < 0.05 (one-way analysis of variance, followed by Tukey’s test for multiple comparisons).
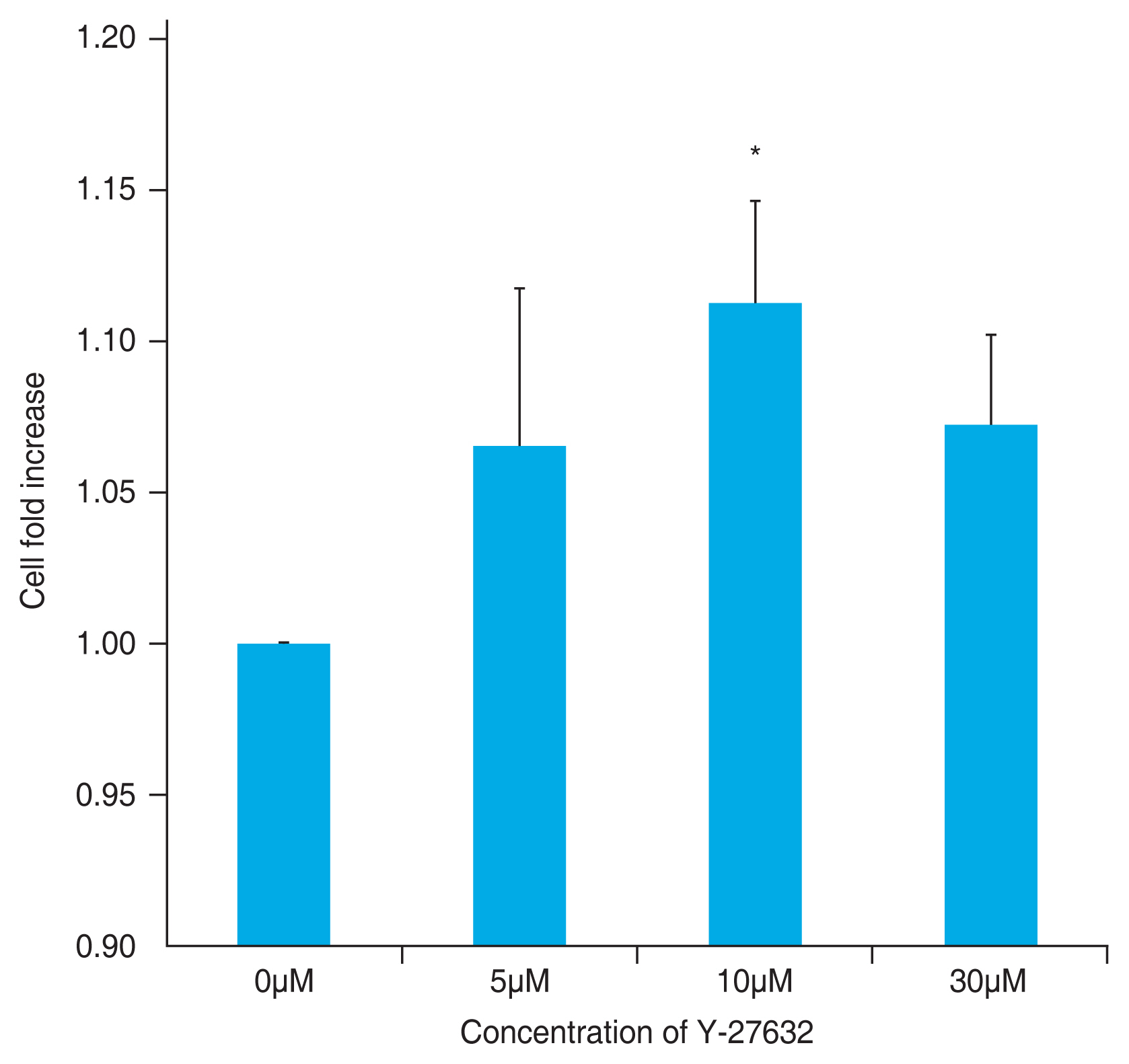
Fig. 6
Morphologic changes in differentiated human corneal endothelial progenitor cells (HCEPs) induced by Y-27632. The differentiated HCEPs were stained with phalloidin in the presence of different concentrations of Y-27632. As the concentration of Y-27632 increased, the differentiated HCEPs lost their polygonal shape, and became more elongated; the actin filament distribution was also altered. Scale bars, 100 μm.
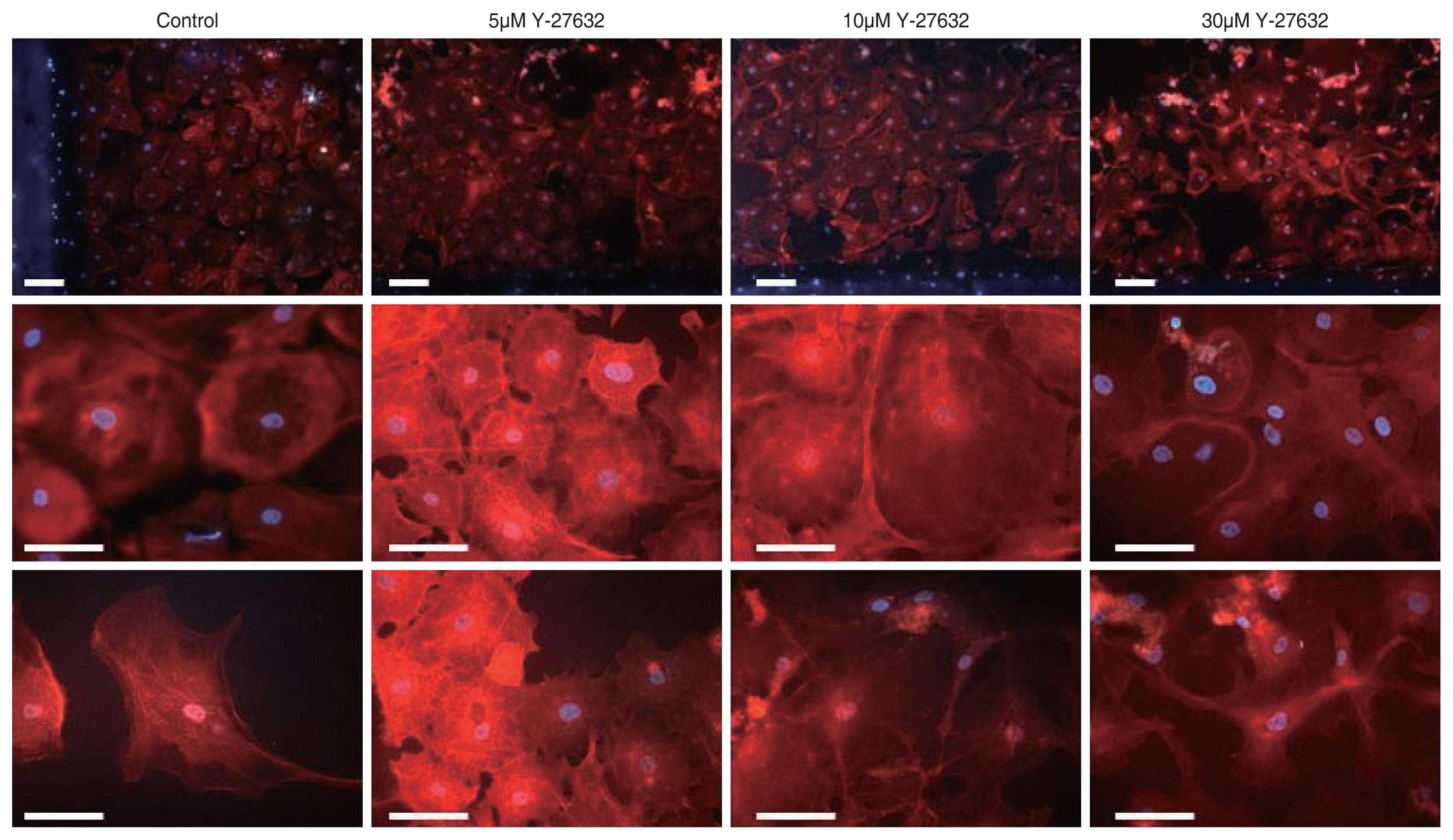
Fig. 7
Enhanced wound-healing ability of differentiated human corneal endothelial progenitor cells (HCEPs) in the presence of Y-27632. (A) Phase-contrast images of differentiated HCEPs in the wound-healing assay in the presence of different concentrations of Y-27632 over 48 hours (scale bars, 300 μm). (B) Kinetics of relative wound closure by differentiated HCEPs treated with different concentration of Y-27632. A significant increase in endothelial wound closure rate was noted when 10μM and 30μM of Y-27632 were added, compared to the control. *p < 0.05 (one-way analysis of variance, followed by Tukey’s test for multiple comparisons).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




