Comparison of Netarsudil/Latanoprost Therapy with Latanoprost Monotherapy for Lowering Intraocular Pressure: A Systematic Review and Meta-analysis
Article information
Abstract
Purpose
Netarsudil is a Rho kinase inhibitor and the first new class of clinically useful ocular hypotensive agents. In this study, we conducted a systematic literature review and meta-analysis to summarize and synthesize the available evidence on the efficacy and safety of fixed-dose combination (FDC) therapy with netarsudil/latanoprost in patients with glaucoma.
Methods
We identified relevant studies in PubMed, Ovid Medline, Embase, and Cochrane Central until April 2021. The quality of the studies and the level of evidence were assessed using the Risk of Bias tool. Efficacy was measured as the mean difference in reducing intraocular pressure (IOP), and safety was assessed by the risk of conjunctival hyperemia (CH) due to FDC therapy, netarsudil monotherapy, or latanoprost monotherapy.
Results
Four studies met the predefined eligibility criteria and were included in the meta-analysis. The mean difference in the reduction in IOP after 2 weeks and 4 to 6 weeks of drug administration was −2.41 mmHg (95% confidence interval [CI], −2.95 to −1.87) and −1.77 mmHg (95% CI, −2.31 to −1.87), respectively, in patients receiving FDC therapy versus those receiving latanoprost monotherapy. On the other hand, latanoprost monotherapy had a greater effect in reducing IOP than netarsudil monotherapy after 4 to 6 weeks of administration (mean difference, 0.95 mmHg; 95% CI, 0.43 to 1.47). The risk of CH was significantly higher with both FDC therapy and netarsudil monotherapy compared to latanoprost monotherapy in week 12, where the relative ratio was 3.01 (95% CI, 1.95 to 4.66) and 2.33 (95% CI, 1.54 to 3.54), each.
Conclusions
Netarsudil/latanoprost FDC therapy has a significantly greater effect on reducing IOP than latanoprost alone. The symptoms of CH were mostly mild, and only a few glaucoma patients discontinued the medication owing to CH in earlier clinical trials. Therefore, it would be beneficial to consider the administration of netarsudil/latanoprost FDC therapy in patients with glaucoma.
Glaucoma is an optic neuropathy that can result in the loss of vision due to the slow progressive degeneration of retinal ganglion cells and their axon. Intraocular pressure (IOP) is the most potent risk factor that contributes to the progression of glaucoma, by causing the death of retinal ganglion cells and optic nerve fibers [1]. Approximately 57.5 million people worldwide are affected by primary open-angle glaucoma (OAG), which is the most common type of glaucoma where the stiffness of the trabecular meshwork increases the resistance to the outflow of aqueous humor [2,3]. Clinical trials have reported that IOP reduction decreases glaucoma progression [4,5]. Thus, the primary goal in the management of glaucoma is to prevent or control elevated IOP.
Pharmacotherapy applied as prescription eye drops is the most common treatment for glaucoma, which works by lowering the pressure in the eyes and reducing the progression of damage to the optic nerve [6]. A wide array of topical antiglaucoma drugs is available, including prostaglandin analogs, β-blockers, carbonic anhydrase inhibitors, alpha-2 agonists, and cholinergic agents [7]. Patients with glaucoma usually begin with a single pharmacotherapy regimen. When monotherapy is not sufficient to lower IOP, combination pharmacotherapy is indicated [7]. However, concomitant use of two different drugs may lower medication adherence and consequently fail to control IOP [8]. Thus, to reduce the number of drugs a person intake and to improve medication adherence, a fixed-dose combination (FDC) therapy (i.e., two or more drugs contained in a single dosage form), which has advantages in patient convenience, has been developed for glaucoma patients who do not respond to monotherapy [9]. Examples of FDC therapies commonly applied to glaucoma patients include a combination of prostaglandin analogs and β-blockers, combinations of alpha-2 agonists and β-blockers, combinations of carbonic anhydrase inhibitors and β-blockers, combinations of cholinergic agents and β-blockers, and combinations of carbonic anhydrase inhibitors and alpha-2 agonists [7].
Recently, a new FDC of netarsudil and latanoprost has been introduced for the reduction of elevated IOP in patients with OAG or ocular hypertension (OHT) [10]. Latanoprost is the most frequently prescribed prostaglandin analog, and it lowers IOP primarily by increasing uveoscleral outflow of aqueous humor [11,12]. It is a well-tolerated drug where 16% patients experienced adverse events such as hyperemia, dryness, or discomfort [13]. Meanwhile, according to the US Food and Drug Administration (FDA) in 2017 and the European Medicines Agency in 2019, netarsudil was the first new class of clinically useful ocular hypotensive agents since the US FDA approval of latanoprost in 1996. Netarsudil is a Rho kinase inhibitor that lowers IOP by increasing the outflow of aqueous humor through the trabecular meshwork, reducing pressure in the veins of the episcleral layer, and inhibiting the norepinephrine transporter [14]. Several clinical trials have been carried out to investigate the efficacy and safety of FDC of netarsudil and latanoprost for OHT in comparison to netarsudil monotherapy [15–18]. Each trial was performed in different environments, used different outcome measures, and yielded different results. With a relatively short history since the FDA approval, evidence on clinical usefulness of this particular FDC is limited. Thus, we conducted a systematic literature review and meta-analysis to summarize the available evidence on the efficacy and safety of FDC therapy of netarsudil and latanoprost in comparison with latanoprost monotherapy. Further, we collected evidence on the comparison between netarsudil and latanoprost monotherapies. Our study results are expected to contribute to improving the health outcomes of glaucoma patients as they provide information on evidence-based drug use.
Materials and Methods
Search strategy
This systematic review and meta-analysis was conducted based on the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) (Supplementary Table 1) [19,20]. We developed a protocol in advance to specify the objective, outcome, eligibility criteria, search strategy, methods for study selection, data extraction, and data synthesis for meta-analysis. Before conducting the literature search, we defined structured research questions following the PICO (population, intervention, comparison, and outcome) format: “In glaucoma or OHT patients (population), is netarsudil/latanoprost FDC therapy (intervention), compared to latanoprost monotherapy (comparison), more effective in lowering IOP and safer from conjunctival hyperemia (CH; outcome)?”; and “In glaucoma or OHT patients (population), is netarsudil monotherapy (intervention), compared to latanoprost monotherapy (comparison), more effective in lowering IOP and safer from CH (outcome)?”
We searched four core databases, including PubMed, Ovid Medline, Embase, and Cochrane Central from April 21 to 23, 2021. The following search terms were used: “glaucoma” and “ocular hypertension” for population; “netarsudil/ latanoprost” and “netarsudil” for intervention; “latanoprost” for comparison; and “intraocular pressure” for outcome. However, to improve the sensitivity of the literature search, search terms for comparison and outcome were not included in the search formula. Search terms belonging to each group (i.e., population and intervention) were combined using “OR,” whereas population and intervention were combined by “AND.”
Study selection
Study selection was independently performed by three reviewers (HSA, JWL, and JC) using a standard extraction form. In the case of disagreement, we selected studies which the majority agreed to select. After identifying the literature based on the predefined search terms, duplicates among the databases were removed. Next, the first screening was carried out based on the titles and abstracts of the studies, and the second screening was performed by reviewing the full text.
The selection criteria for studies were as follows: (1) the population of interest was patients with glaucoma or OHT, (2) the treatment of interest included netarsudil monotherapy or FDC therapy of netarsudil/latanoprost, (3) the treatment of interest included latanoprost monotherapy, (4) the reported outcome measures included IOP, and (5) the presented original data were clinical trials. Further, we excluded grey documents, duplicates, and documents written in languages other than English. The study selection process is illustrated in Fig. 1.
Data extraction and quality assessment
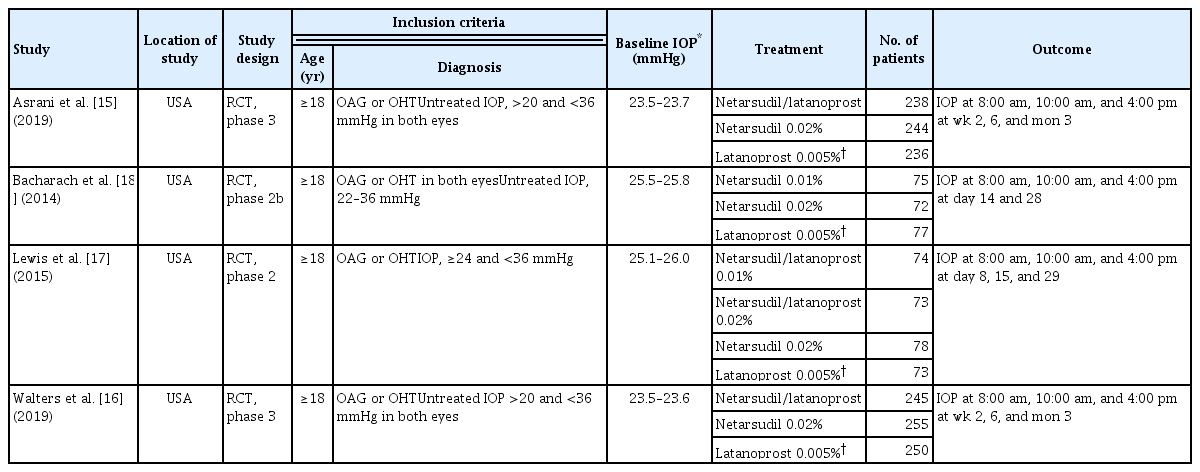
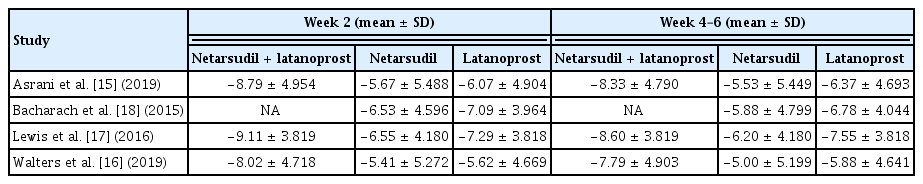
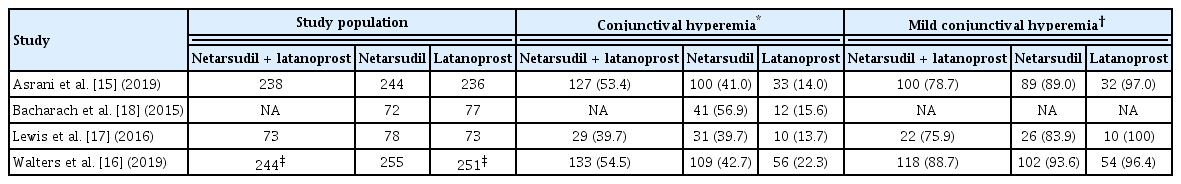
From the selected studies, three reviewers (HSA, JWL, and JC) independently extracted the name of the first author, year of publication, location of the study conducted, study design, inclusion criteria of the study population, intervention, outcome measurements, and funding source. In addition, we independently extracted efficacy data, which is the reduction in IOP after medication, and safety data, the number of patients with CH. The characteristics, efficacy data, and safety data of the selected studies are summarized in Tables 1, 2, and 3, respectively [15–18].
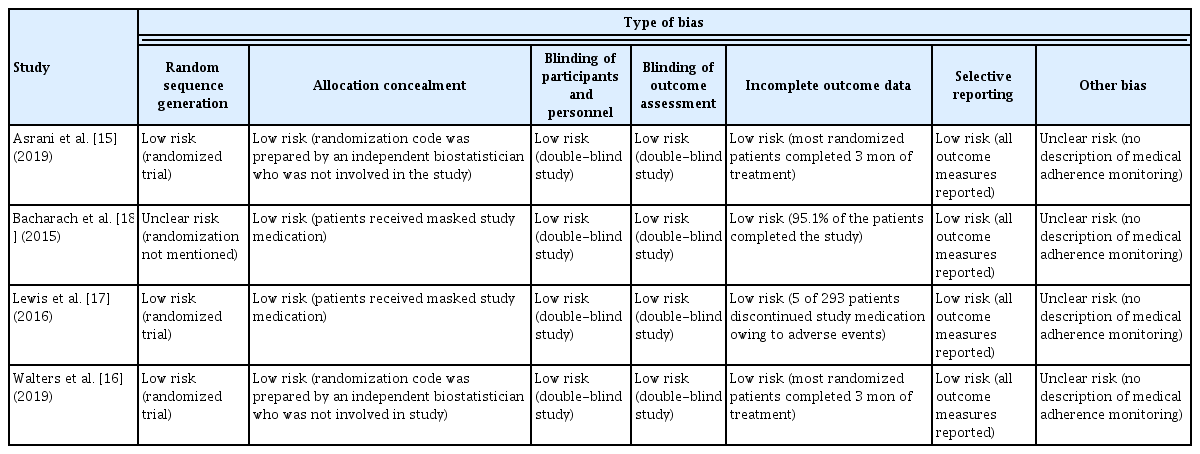
The quality of the selected studies was evaluated using the Risk of Bias ver. 1.0 (Cochrane Collaboration, Copenhagen, Denmark) (Table 4). The Risk of Bias is a tool for assessing the risk of bias in randomized comparative clinical trials. It consists of seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases [21]. For each domain, the risk of bias was evaluated as high, low, and unclear according to the contents written in the study. Three independent researchers (HSA, JWL, and JC) evaluated the quality of the literature, and disagreements were resolved by reaching a consensus through mutual discussion.
Outcome measures and statistical analysis
1) Efficacy
For each study selected for our analysis, we assessed the IOP-lowering effect of each study drug (i.e., netarsudil/latanoprost FDC therapy, netarsudil monotherapy, or latanoprost monotherapy) by evaluating the “reduction in IOP” after medication. Only the subjects who received 0.02% netarsudil were included in the meta-analysis. Patients using ocular hypotensive medications were required to undergo a washout before study entry: 4 weeks for prostaglandin analogs and β-adrenergic antagonists, 2 weeks for adrenergic agonists, and 5 days for muscarinic agonists and carbonic anhydrase inhibitors.
“Reduction in IOP at 2 weeks after medication” was measured by subtracting IOP recorded at 2 weeks after medication from IOP at baseline (i.e., day 0 after washout and before study medication). A negative value of reduction implies that the study drug is effective in lowering IOP. “Reduction in IOP at 4 to 6 weeks after medication” was measured in the same manner. From each study, we extracted the mean difference in the reduction in IOP at 2 weeks or at 4 to 6 weeks with netarsudil/latanoprost FDC therapy versus latanoprost monotherapy, or netarsudil monotherapy versus latanoprost monotherapy, in order to collect comparative effectiveness data. All four studies were used to synthesize IOP reduction data in netarsudil monotherapy versus latanoprost monotherapy, and all studies except Bacharach et al. [18] were used to synthesize IOP reduction data in FDC therapy versus latanoprost monotherapy.
To calculate the confidence interval (CI) for the population mean of IOP reduction, we need a population standard deviation (SD). All four articles selected from the literature search recorded IOP three times a day: at 8:00 am, 10:00 am, and 4:00 pm. Lewis et al. [17] provided the SD for the mean value of the three IOPs measured at different times of the day, whereas the other three articles provided separate mean and SD for IOP at 8:00 am, 10:00 am, and 4:00 pm, respectively. Thus, for calculating CI, we used the mean IOP of the three measurements and its SD for the study by Lewis et al. [17] and used the mean IOP measured at 10:00 am and its SD for the other three articles [17]. Because 10:00 am was placed in the middle of the three time points, we selected IOP at 10:00 am as the representative value, assuming that it would be similar to the mean IOP of the three measurements.
The timing and frequency of IOP measurements after the initiation of medication varied across the studies. IOP at 2 weeks after medication was recorded by all four articles, IOP at 4 and 6 weeks after medication was recorded in two articles. Lewis et al. [17] and Bacharach et al. [18] recorded IOP at week 4, and Asrani et al. [15] and Walters et al. [16] recorded IOP at week 6.
We conducted a meta-analysis using the mean IOP and its SD at week 2 in all four studies. In the study by Lewis et al. [17], the SD of the mean IOP at week 2 was not provided. As it was observed that the SD of mean IOP at weeks 2 and weeks 4 to 6 were similar in the other three articles, we used the SD at week 4 as an alternative to SD at week 2 for the study by Lewis et al. [17]. We conducted a meta-analysis using IOP at weeks 4 and 6. We assumed that this would not produce biased results because the mean IOP and its SD measured at weeks 4 and 6 were similar in each study.
2) Safety
To compare the safety of each drug, we calculated the relative risk of CH, the most frequent adverse event of IOP-lowering drugs. CH is a common ocular symptom caused by a pathological vasodilatory response due to inflammation. To evaluate the severity of CH, biomicroscopic grading was carried out as of 8:00 am. Mild symptom was defined as “prominent pinkish-red color of both the bulbar and palpebral conjunctiva,” moderate as “bright, scarlet red color of the bulbar and palpebral conjunctiva,” and severe as “beefy red with petechiae; dark red bulbar and palpebral conjunctiva a with evidence of subconjunctival hemorrhage” [15–17]. The relative risk of CH was calculated as follows:
3) Statistical analysis
A generic inverse-variance estimation method and a random- effects model were used to conduct a meta-analysis for both efficacy and safety. The inverse-variance method, which uses the reciprocal of the variance of the effect estimate as the weight of each study, is the most commonly used effect estimation method in meta-analysis [22]. The random-effect model assumes that there is no single true value of the intervention effect in individual studies and that it follows a normal distribution centered on the average value of the intervention effect [22]. To evaluate the heterogeneity between studies, we calculated the Q value and conducted a chi-square test. In addition, we calculated I2, which represents the ratio of variance between studies. If the selected studies included more than 10 studies, the test for publication bias was carried out by interpreting funnel plots [23]. All analyses were performed using the Review Manager ver. 5.3 (Cochrane Collaboration), and the analysis results were visually confirmed through a forest plot.
Results
Search results
Fig. 1 presents a flowchart for the identification of relevant studies. Of the 375 potentially relevant studies identified, 163 duplicates were excluded. Screening based on titles and abstracts excluded 175 studies, mainly because they were not clinical trials (n = 71) or did not include netarsudil as the treatment of interest (n = 37). Full-text assessment excluded 33 studies, and finally, four studies met the eligibility criteria and were included in the qualitative and quantitative synthesis [15–18]. As the selected studies had fewer than 10 studies, we did not test for publication bias because the power was too weak for interpretation of the funnel plot [23].
Study characteristics
Table 1 summarizes the main characteristics of the four articles included in this study. They were all randomized controlled trials, two of which were phase II trials and the other two were phase III trials. All studies were conducted in glaucoma patients aged 18 years or older. Patients with a severe untreated IOP above 36 mmHg were excluded. Three studies evaluated and compared the efficacy and adverse events of netarsudil/latanoprost FDC therapy, netarsudil 0.02%, and latanoprost 0.005% [15–17]. Bacharach et al. [18] compared two monotherapies, netarsudil and latanoprost. All studies were consistent in the timing of IOP measurement during the day: 8:00 am, 10:00 am, and 4:00 pm. However, there was a variation in the period of eye drop administration before measuring IOP. In the studies by Asrani et al. [15] and Walter et al. [16], IOP was measured at 2 weeks, 6 weeks, and 3 months after the initiation of medication. In the study by Bacharach et al. [18], it was measured at 2 and 4 weeks. In the study by Lewis et al. [17], it was measured at 1, 2, and 4 weeks. All four clinical trials performed efficacy analyzes on the intent-to-treat cohort.
The risk of bias for each article is presented in Table 4. Randomization was performed properly, and the risk of bias of random sequence generation items was low in three articles [15–17]. However, although they mentioned that randomization was performed, Bacharach et al. did not provide a specific method, and this was marked as unclear risk [18]. In all four studies, double blinding was performed well, there were only a few dropouts, and all outcome values were reported properly. Therefore, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting were evaluated as low risk. Lastly, as there was no description of medical adherence monitoring in all four studies, other biases were determined as unclear risk.
Main analysis and subgroup analysis
1) Reduction in intraocular pressure
A meta-analysis was performed on three studies that provided information on the efficacy of netarsudil/latanoprost FDC therapy and latanoprost monotherapy [15–17]. Combining the three studies, there were 555 patients administered netarsudil/latanoprost FDC therapy and 559 patients receiving latanoprost monotherapy. The difference from baseline in IOP, measured after 2 weeks and 4 to 6 weeks from the start date of eye drop administration, was analyzed. The results are presented as a forest plot in Fig. 2. The mean difference in the reduction in IOP after 2 weeks of combination therapy was −2.41 mmHg (95% CI, −2.95 to −1.87) lower than that after monotherapy, which can be explained that the FDC therapy has a greater effect on reducing IOP. There was no heterogeneity between studies (I2 = 0%), and the forest plot showed that netarsudil/latanoprost FDC therapy was more effective in reducing IOP than latanoprost monotherapy in all clinical trials. The mean difference in the reduction in IOP measured at 4 to 6 weeks after drug administration was −1.77 mmHg (95% CI, −2.31 to −1.23) in patients receiving combination formulation vs. latanoprost monotherapy. There was no heterogeneity between studies (I2 = 0%). These results suggest that netarsudil/latanoprost FDC therapy was significantly more effective in reducing IOP than latanoprost alone, regardless of the duration of medication when used for up to 6 weeks.

Decreases in intraocular pressure with netarsudil/latanoprost fixed-dose combination therapy versus latanoprost monotherapy. SD = standard deviation; IV = interval variable; CI = confidence interval.
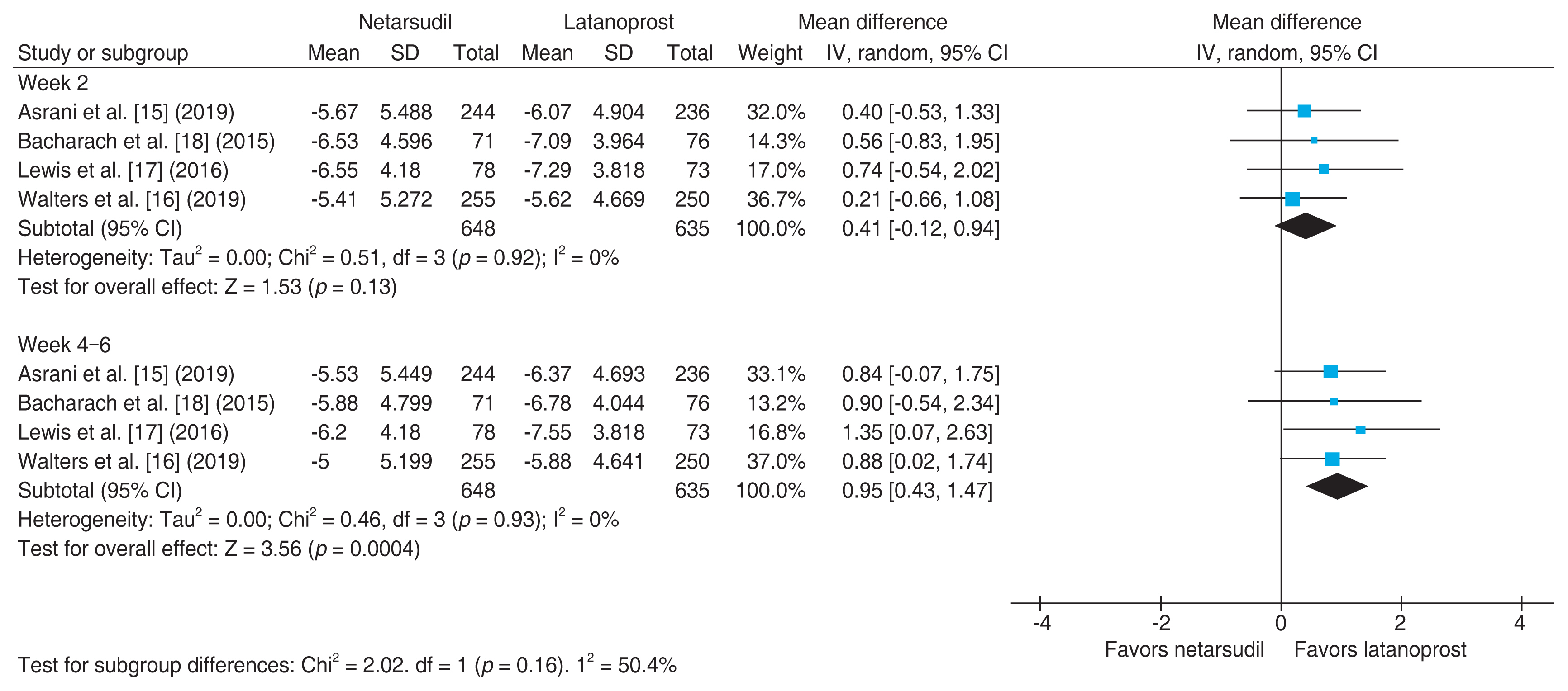
The forest plot in Fig. 3 shows the results of meta-analysis comparing the efficacy of monotherapy with netarsudil versus latanoprost at weeks 2 and 4 to 6 after medication [15–18]. A total of 648 patients were administered netarsudil, and 635 patients received latanoprost. The mean difference in the reduction in IOP after 2 weeks of drug administration was 0.41 mmHg (95% CI, −0.12 to 0.94) with netarsudil versus latanoprost. This implies that the reduction of IOP was greater by 0.41 mmHg in patients treated with latanoprost than in those with netarsudil, but the difference was not significant (p = 0.13). There was no heterogeneity between the studies (I2 = 0%), and all four articles showed that latanoprost had a greater effect than netarsudil, although the difference was not significant in any of the studies. When IOP was measured at 4–6 weeks after the start of drug administration, the reduction in IOP was greater by 0.95 mmHg with latanoprost monotherapy (95% CI, 0.43 to 1.47), with significance at 5%.
2) Safety
The occurrence rate of CH, which is a common side effect of both netarsudil and latanoprost monotherapies, was analyzed [15–18,24]. Fig. 4 presents the results of meta-analysis based on the two studies comparing netarsudil/ latanoprost FDC therapy and latanoprost monotherapy for up to 12 weeks [15,16]. The risk ratio was 3.01 (95% CI, 1.95 to 4.66), which means that the risk of CH was significantly higher with the combination therapy by threefold. Although the high I2 statistic (76%, p = 0.04) suggests heterogeneity between the two studies, the forest plot confirms that the risk of CH was consistently higher in patients who received netarsudil/latanoprost FDC therapy than in those who received latanoprost monotherapy in both studies (Fig. 4).

Risk ratio of conjunctival hyperemia with netarsudil/latanoprost fixed-dose combination therapy versus latanoprost monotherapy. IV = interval variable; CI = confidence interval.
Fig. 5 shows the results of meta-analyses comparing the risk of CH between netarsudil and latanoprost monotherapies. Subgroup analysis was performed according to medication period. When the drug was administered for 4 weeks, the risk ratio of netarsudil and latanoprost monotherapies was 2.97 (95% CI, 1.94 to 4.55), indicating 2.97 times higher risk of hyperemia among patients treated with netarsudil [17,18]. Similar results were obtained for a medication period of 12 weeks, with a 2.33 times (95% CI, 1.54 to 3.54) higher risk of CH with netarsudil monotherapy [15,16]. Although a significant heterogeneity was shown between the two studies (I2 = 72%, p = 0.06), the trend of higher risk among those treated with netarsudil was consistent in the two studies, with risk ratios of 2.93 (95% CI, 2.06 to 4.16) and 1.92 (95% CI, 1.46 to 2.51), respectively.
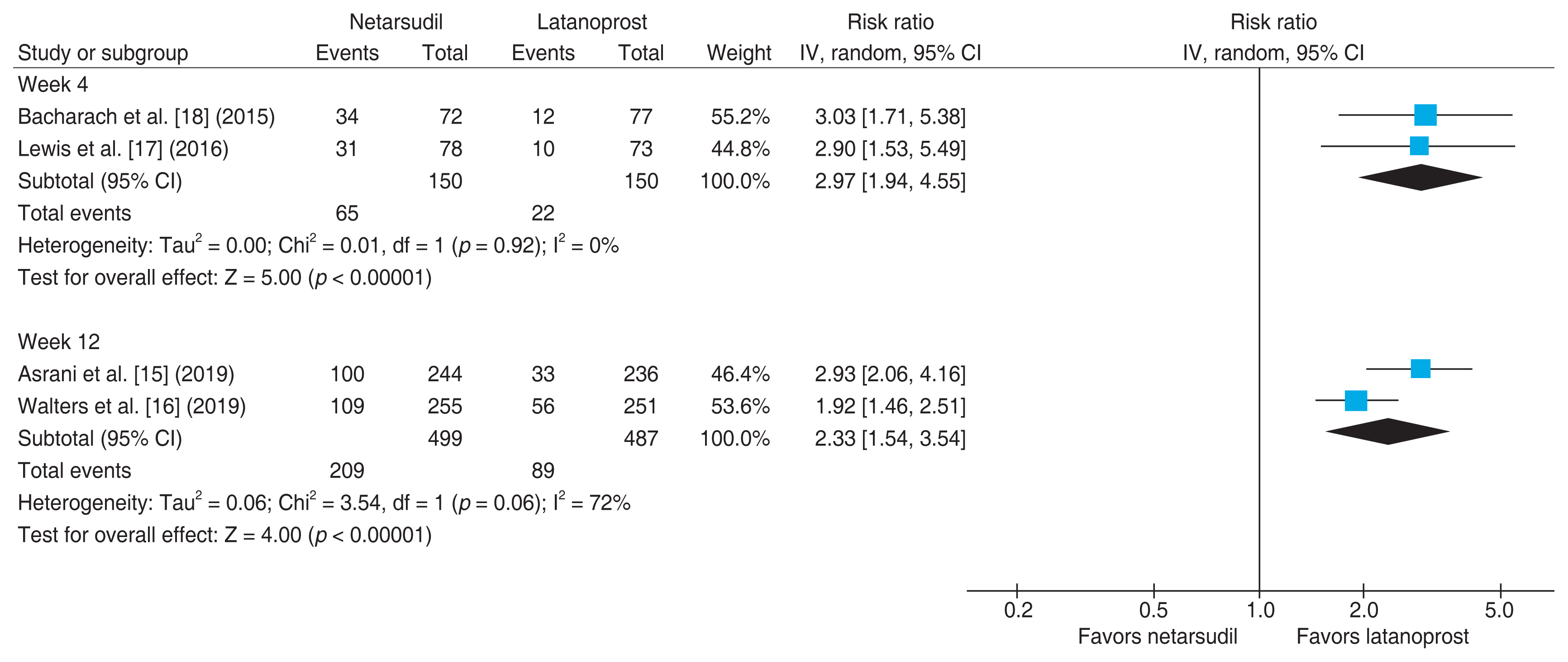
Risk ratio of conjunctival hyperemia with netarsudil versus latanoprost monotherapies. IV = interval variable; CI = confidence interval.
The drop-out rates of FDC, netarsudil monotherapy, and latanoprost monotherapy because of CH were 7.1% (17 of 238), 4.9% (12 of 244), and 0% (0 of 236), respectively in Asrani et al. [15], and 2.5% (6 of 244), 2.0% (5 of 255), and 0.4% (1 of 251) respectively in Walters et al. [16]. According to Asrani et al. [15], Lewis et al. [17], and Walters et al. [16], 78.7%, 75.9%, and 88.7% of patients who experienced CH due to FDC exhibited mild symptoms, respectively. In addition to FDC, 89.0%, 83.9%, and 93.6% of patients treated with netarsudil monotherapy as well as 97.0%, 100%, and 96.4% of patients treated with latanoprost monotherapy had mild symptoms (Table 3).
Discussion
In the present study, we investigated the efficacy and safety of netarsudil/latanoprost FDC therapy. Our results suggested that netarsudil/latanoprost FDC therapy had an additional IOP reduction effect compared to latanoprost monotherapy, but also significantly increased the risk of mild CH.
Netarsudil and latanoprost employ different mechanisms for reducing IOP. Netarsudil acts as a Rho-associated protein kinase (ROCK) inhibitor. The ROCK signaling pathway is an important signal transduction system, in which activated ROCK phosphorylates substrates such as myosin light chain and LIM kinase, resulting in the inhibition of central nervous system regeneration [25,26]. Inhibition of the ROCK pathway by netarsudil administration to the eye results in the relaxation of the trabecular meshwork and increases aqueous humor outflow, thereby reducing IOP. It is also involved in optic nerve neuroprotection via improving retinal ganglion cell survival and promoting retinal ganglion cell axon regeneration [27]. In contrast, latanoprost is a prostaglandin analog. It increases the biosynthesis of matrix metalloproteins by acting on the prostaglandin F2α receptor. Matrix metalloproteins increase the space between the ciliary muscle fibers, thereby reducing the resistance of the uveoscleral outflow pathway and promoting aqueous humor outflow [28].
Compared to latanoprost monotherapy, netarsudil/latanoprost FDC therapy can have an additional IOP-lowering effect owing to the two separate mechanisms of action: netarsudil as a ROCK inhibitor and latanoprost as a prostaglandin analog. In a clinical trial involving 255 patients with primary OAG, a reduction in IOP by 1 mmHg reduced the risk of optic nerve damage by 10% [29]. The additional reduction of 2.41 mmHg (95% CI, −2.95 to −1.87) and 1.77 mmHg (95% CI, −2.31 to −1.23) in IOP by netarsudil/ latanoprost FDC therapy may be effective in slowing down the damage of retinal ganglion cells and optic nerve, delaying the vision loss associated with glaucoma.
CH is a typical adverse effect of netarsudil/latanoprost FDC therapy. It is a conjunctival reaction that appears as dilation and redness of the conjunctival vessels, causes discomfort and itching, and may decrease medication adherence [30]. Our results showed that netarsudil/latanoprost FDC therapy was associated with a higher risk of CH (relative risk, 3.01; 95% CI, 1.95 to 4.66). However, most of the symptoms were mild, and only a few patients discontinued the treatment. According to the clinical results of Asrani et al. [15], 85.8% of patients with CH showed mild symptoms, and only 7.1% of patients (n = 17) taking netarsudil/latanoprost FDC discontinued the medication because of this condition. In addition, according to the clinical results of Walters et al. [16] and Lewis et al. [17], among patients with CH, the proportion of patients with mild symptoms was 88.7% and 76%, respectively, and drug discontinuation due to CH was 2.5% (n = 6) and 0% (n = 0), respectively [16,17].
Netarsudil/latanoprost FDC can be prescribed to patients whose IOP is high despite the use of latanoprost monotherapy. In a clinical trial involving 340 patients with primary OAG or OHT, IOP in 4.1% of patients did not decrease by more than 15% from baseline despite the administration of latanoprost [31]. In another study, latanoprost administration in five out of 20 patients (25%) did not reduce IOP by more than 20% [32]. In such patients, changing the treatment to netarsudil/latanoprost FDC would be effective for treating glaucoma. Our results showed that netarsudil monotherapy was less effective than latanoprost monotherapy and had a higher risk of CH. Therefore, if netarsudil is considered a treatment choice for glaucoma patients, combining it with latanoprost would be more beneficial than monotherapy.
This study had several limitations. First, there was a difference in mean IOP used. Asrani et al. [15], Walters et al. [16], and Bacharach et al. [18] presented the mean and SD of IOP measured at 8 am, 10 am, and 4 pm, respectively, whereas Lewis et al. [17] presented the average daily IOP and SD regardless of time. Therefore, in the meta-analysis, we selected the daily mean IOP for study by Lewis et al. [17] and the mean IOP measured at 10 am for the remaining three studies. In addition, in the study by Lewis et al. [17], the SD of the IOP measured at week 2 was not presented, so we assumed that it would be the same as the SD of the IOP measured at week 4. However, as the heterogeneity of all the meta-analyses, including study by Lewis et al. [17], was reported as 0%, the effect of this difference on the results seemed to be negligible. Second, the time of IOP measurement was different for each study in the 4 to 6 weeks analysis. Asrani et al. [15] and Walters et al. [16] measured IOP after 6 weeks, whereas Lewis et al. [17] and Bacharach et al. [18] measured IOP after 4 weeks. As the IOP and SD measured at the 4th and 6th weeks of each study were similar, the results were grouped into 4 to 6 weeks for meta-analysis. As a result, the heterogeneity was 0%, suggesting that grouping weeks 4 and 6 had a negligible effect on the results. Third, because all the selected studies were funded by Aerie Pharmaceutical Inc. (Durham, NC, USA), a pharmaceutical company that developed Rhopressa (netarsudil) and Rocklatan (netarsudil/ latanoprost), caution is needed when interpreting the results.
In conclusion, netarsudil/latanoprost FDC therapy is a more effective treatment than latanoprost monotherapy for decreasing IOP in glaucoma patients. A higher risk of CH associated with netarsudil/latanoprost FDC therapy may not be an obstacle for the treatment of glaucoma patients, as the symptoms are mild and rarely lead to medication discontinuation. Therefore, it would be beneficial to consider the administration of netarsudil/latanoprost FDC therapy in patients with glaucoma. Additionally, follow-up studies are needed to investigate the efficacy and safety of netarsudil/latanoprost FDC therapy in glaucoma patients who failed to respond to other types of medications, or those who are resistant to latanoprost.
Supplementary Materials
Supplementary materials are available at https://doi.org/10.334/kjo.2022.0061.
Supplementary Table 1. PRISMA 2020 checklist
kjo-2022-0061-suppl.pdfAcknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: None.