Effects of Glaucoma Medication on Dry Eye Syndrome and Quality of Life in Patients with Glaucoma
Article information
Abstract
Purpose
To investigate ocular surface diseases and changes in the quality of life of patients using glaucoma medications.
Methods
Participants were divided into the normal (31 individuals, 62 eyes) and glaucoma medication (30 patients, 60 eyes) groups. Changes in tear break-up time, lipid layer thickness (LLT), corneal and conjunctival staining scores, ocular surface disease index (OSDI), and Visual Function Questionnaire 25 (VFQ-25) score were assessed for 1 year.
Results
The change in mean LLT was lower in glaucomatous eyes than in control eyes (p = 0.019) after 1 year. The results of OSDI deteriorated (p’ = 0.008), but conjunctival staining and Schirmer test results showed improvement in glaucomatous eyes compared to those in control eyes (p’ =0.035 and 0.009, respectively). The average LLT decreased at 6 and 12 months, but there was no change at 24 months. In pairwise analysis, the decrease in LLT over the first 6 months was statistically significant (p < 0.001) and remained unchanged until 24 months. Among the VFQ items, scores for near activity and social function deteriorated over 1 year in the medication group (p’ = 0.033 and 0.015, respectively). However, there was no difference in the total VFQ score.
Conclusions
Significant reduction in LLT and deterioration of OSDI were observed in the medication group compared to the control group. However, this deterioration was observed only in the first 6 months. There was no significant difference in the VFQ total score. Nonetheless, there were significant differences in near activity and social function between the control and medication groups. Therefore, the results of this study showed that although glaucoma medication worsened eye dryness, the change was limited and did not worsen the quality of life. Glaucoma medication should be used with the consideration that they can limit near activity and social functioning.
Glaucoma is a progressive optic neuropathy and the best primary treatment for this disease is anti-glaucoma medication therapy [1,2]. However, the active ingredients and preservatives of these medications can cause chronic inflammation of the ocular surface in patients with glaucoma, which can lead to structural changes in the meibomian gland [1–3]. Ocular surface diseases in patients with glaucoma can reduce glaucoma medication compliance and quality of life (QOL) [4–6].
Rossi et al. [6] reported that patients using glaucoma medication showed more frequent symptoms of dry eye syndrome compared to participants not on glaucoma medication. They also reported that eye dryness negatively affected a patient’s QOL. Camp et al. [4] also observed that an increased number of glaucoma medications exacerbated eye dryness, leading to poor QOL. Although previous studies including the aforementioned results have reported that glaucoma medications are related to ocular surface disease [4,6–8], studies on how changes in ocular surface disease affect the QOL of patients with glaucoma are relatively limited.
Therefore, this study aimed to investigate the subjective symptom changes using tear break-up time (TBUT), lipid layer thickness (LLT), corneal and conjunctival stain scores, and ocular surface disease index (OSDI) in patients using glaucoma medications. Additionally, the overall QOL was observed using the Visual Function Questionnaire 25 (VFQ-25). We analyzed the changes in ocular surface disease and QOL in patients using glaucoma medication, including a fixed combination of timolol and dorzolamide, and latanoprost, which are widely administered by clinicians.
Materials and Methods
Participants and clinical examination
This prospective study was approved by the institutional review board of Yonsei University Severance Hospital (4-2017-0633). All research methods adhered to the tenets of the Declaration of Helsinki and written informed consent was obtained from all participants. All participants were examined at the glaucoma clinic of the Department of Ophthalmology at Severance Hospital, Yonsei University School of Medicine, Seoul, Korea.
We included 37 patients diagnosed with glaucoma or suspected with glaucoma but were not on glaucoma medications and 30 patients who used glaucoma eye drops. We investigated the changes in TBUT, LLT, corneal and conjunctival staining scores, and OSDI over 1 year in all participants in each group. The VFQ-25 was also used to analyze changes in the QOL of all participants.
Assessment of open-angle glaucoma
Screening for open-angle glaucoma was performed using gonioscopy, red-free photography (VISUCAM 200; Carl Zeiss Meditec AG, Jena, Germany), Cirrus HD optical coherence tomography (Carl Zeiss Meditec AG), and a visual field test (24-2 Swedish Interactive Threshold Algorithm, Humphrey Visual Field Analyzer; Carl Zeiss Meditec AG). Healthy eyes were defined as eyes with intraocular pressure of ≤21 mmHg with no history of elevated intraocular pressure, normal-appearing optic discs, intact neuroretinal rims and peripapillary retinal nerve fiber layers, normal visual field test results (defined as a pattern standard deviation within 95% confidence intervals), and glaucoma hemifield test results within normal limits. Glaucoma was defined as the occurrence of glaucomatous optic nerve head changes (e.g., vertical cup-to-disc ratio >0.7, focal or diffuse neural rim loss, disc hemorrhage, or retinal nerve fiber layer defects on red-free photography), regardless of the intraocular pressure value, and compatible glaucomatous visual field defects [9].
Participants who had been using topical anti-glaucoma medication were excluded from the open-angle glaucoma group. We set a limit on the types of medications to minimize the bias caused by varying types of anti-glaucoma medication; only participants prescribed with a fixed combination of timolol and dorzolamide or latanoprost were enrolled in this study. A fixed combination of timolol and dorzolamide and latanoprost are generally the most commonly prescribed drugs in glaucoma patients, and are also the most commonly prescribed drugs in the institutions where this study was conducted [10,11]. All types of containers, bottle-type medication with preservatives or single-use medication vials, were allowed for both medications. Those prescribed both medications (latanoprost, a fixed combination of timolol, and dorzolamide) were excluded.
Assessment of ocular surface status
Examinations were performed in the following order: LLT measurement using an interferometer, Schirmer test, TBUT assessment, and ocular surface staining (Oxford grading scale). These examinations were performed by a single examiner (KL) at an interval of at least 10 minutes. Patients were required to visit the hospital at the same time for examination at each visit.
TBUT is a measure of the interval between the last blink and the tear break-up. After gently applying a sodium fluorescein dye strip to the palpebral conjunctiva, the tear disruption time was identified using a blue filter [9,12]. The Schirmer test was used to measure tear secretion. First, proparacaine 0.5% anesthetic eye drops were applied to the patient, and after 1 minute, the test strip was placed on 1/3 of the eyelid below. After 5 minutes, the length of the moistened area of the test strip was measured [12].
The subjective symptoms of the ocular surface disease were evaluated using OSDI [13]. The OSDI questionnaire consists of questions related to ocular pain, visual difficulties, and environmental factors related to dryness. The Oxford scale was used in this study to measure corneal and conjunctival stains. The Oxford scale was designed to quantify the degree of epithelial damage to the cornea and conjunctiva. After the instillation of the fluorescein dye, a slit-lamp test was used to check the degree of erosion of the cornea and conjunctiva surfaces. The grade was divided from 0 to 5 (from absent to severe) [14].
LipiView (TearScience Inc., Morrisville, NC, USA), a device that measures the LLT using an interferometer, can be used to identify structural changes and dysfunction in the meibomian gland [15]. At our clinic, LLT measurement using an interferometer is routinely performed for patients visiting the hospital with obstructive-type meibomian gland morphology during a slit-lamp examination. The LipiView measures the mean, maximum, and minimum LLT at 20 seconds, and the number of incomplete blinks and total blinks at 20 seconds intervals. Interference color units were used to measure the LLT, with one interference color units corresponding to 1 nm [15,16].
Participants who had used any topical anti-inflammatory, antibiotic, or other medication known to affect the ocular surface, except for anti-glaucoma medications used by glaucoma patients, within 3 months before enrollment in this study were excluded. Participants with a history of artificial tear drop usage within 3 months prior to the study were also excluded. This is because the use of artificial tears may cause bias when measuring tear LLT. Nonetheless, excluding these participants may have shifted the characteristics of the included sample toward less severe dry eye. Only carboxymethyl cellulose was allowed during the study period in case artificial tears were needed. Participants with an uncorrected distant visual acuity worse than 20 / 30, history of ocular surgery, contact lens use within 6 months, or systemic disease related to OSD were also excluded. In addition, to minimize the impact of topical medication as a confounding factor in the measurement of LLT, tests were conducted at least 4 hours after the use of topical eye drops. The temperature of the clinic was maintained at 18°C to 20°C, and the humidity was set at 55%.
Assessment of vision-related QOL
The National Eye Institute Visual Function Questionnaire (NEI-VFQ) 25 is a short version of the NEI-VFQ designed to evaluate the vision-related QOL, reducing the number of existing 51 questions to 25. The NEI-VFQ 25 questionnaire is the most widely used version for studying vision-related QOL in patients with glaucoma [17]. The NEI-VFQ 25 survey was conducted at each visit to measure changes in the overall QOL of the study participants.
Statistical analyses
R ver. 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/) was used to perform all statistical analyses. Differences between the groups in terms of continuous and categorical parameters were compared using independent two-sample t-tests and chi-square tests, respectively. Random effects were set and calibrated for the analysis of both eyes using statistical methods.
The differences in the variables between the initial visit and after 1 year were measured by one-way repeated measure analysis of variance (ANOVA) in normal and glaucomatous eyes, respectively. The differences in the amount of change between the normal and glaucomatous eyes were analyzed using two-way repeated-measures ANOVA. Variables that differed significantly between the normal and glaucoma groups in the previous analyses were adjusted using two-way repeated-measures ANOVA. Statistical significance was defined at p < 0.05.
Results
Demographics
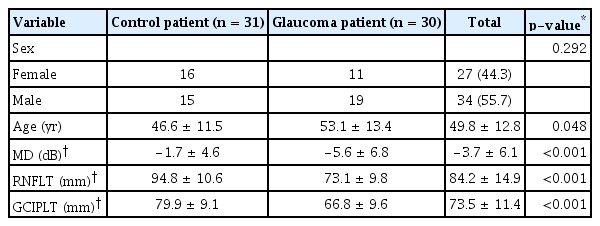
This study included 67 participants. Two participants in the control group refused to participate in the study, and four participants in the control group were lost to follow-up. Therefore, a total of 62 eyes of 31 participants without glaucoma and 60 eyes of 30 participants with glaucoma were investigated. The control group included patients who were suspected of glaucoma or early open-angle glaucoma and were under progress observation without glaucoma medications. No economic compensation was provided to the participants. A fixed combination of timolol and dorzolamide was prescribed to 20 eyes of 10 participants, while latanoprost was prescribed to 40 eyes of 20 participants. The average ages of the participants without and with glaucoma were 46.6 ± 11.5 years and 53.1 ± 13.4 years (p = 0.048), respectively. The proportion of men and women did not differ between the two groups (15 / 31 [48.4%] vs. 19 / 30 [63.3%]; p = 0.359). The eyes of participants with glaucoma had lower mean deviation, thinner retinal nerve fiber layer thickness, and thinner ganglion cell-inner plexiform layer thickness than did the eyes of normal participants (Table 1).
Sex and age showed no statistically significant differences between timolol/dorzolamide and latanoprost. The eyes of those using latanoprost were shown to have lower mean deviation, thinner retinal nerve fiber layer thickness, and thinner ganglion cell-inner plexiform layer thickness than those using timolol/dorzolamide (Table 2).
Effects of glaucoma medication on dry eye
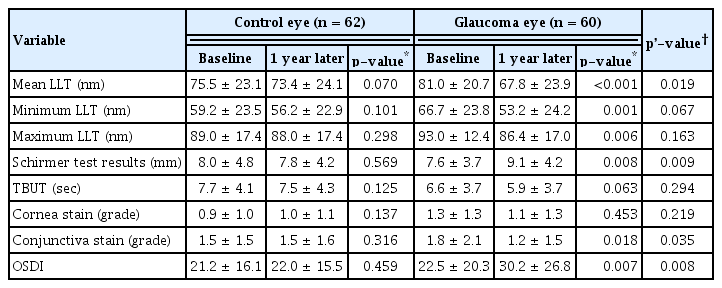
At the 1-year follow-up, the mean LLT was lower in glaucomatous eyes than in control eyes (73.4 ± 24.1, 67.8 ± 23.9, respectively; p’ = 0.004). The OSDI results showed deterioration (p’ = 0.019). However, the conjunctival stain grade and Schirmer test results showed improvement (p’ = 0.035 and 0.009, respectively) in glaucomatous eyes compared to those in control eyes. TBUT also showed deterioration in glaucomatous eyes compared to control eyes. However, these results were not statistically significant (Table 3). When comparing the two glaucoma medication groups, it was observed that mean LLT and minimum LLT were significantly lower in the latanoprost subgroup than in the timolol/dorzolamide subgroup, which was statistically significant (Table 4).
For the subgroup analysis, the mean LLT in participants with glaucoma was monitored for up to 24 months. The average LLT decreased at 6 and 12 months, but there was no change at 24 months compared to that at 12 months, which was statistically significant (Table 5). In pairwise analysis, the decrease in LLT over the first 6 months was statistically significant (p < 0.001). However, it remained unchanged until 24 months. No statistical significance was observed between 6 and 12 months (p = 0.926) between 12 and 24 months (p = 0.742).
Effect of glaucoma medication on vision functions-related QOL
The VFQ of social functioning and near activities scores were lower in the glaucoma group than in the normal group. However, the total VFQ score did not differ significantly between the groups (Table 6). Subgroup analysis by medication showed that there was no statistically significant difference between the two glaucoma medications (Table 7).
Discussion
This study analyzed changes in the ocular surface and QOL of patients who had used glaucoma medication for 1 year. Eyes that were administered glaucoma medications showed decreased LLT and worsened subjective symptoms, such as deterioration of OSDI after 1 year. VFQ-25 tests conducted to measure the overall QOL of participants showed deterioration in near activity and social functioning, but showed no statistically significant deterioration in total scores.
In a previous study, glaucoma medication use was reported to reduce the LLT [8,18]. Lee et al. [8] analyzed the effects of LLT and glaucoma medication and reported a significantly lower LLT in patients with open-angle glaucoma using glaucoma medications than in the control group without glaucoma medications. Ramli et al. [19] reported a high correlation between a lower LLT and an increasing number of glaucoma medications, while Arita et al. [20] showed a high correlation between a lower LLT and a longer duration of glaucoma medication use. However, in the study by Arita et al. [20], the relationship between duration and meibomian gland alternation was not statistically significant in multivariate analysis. Arita et al. [20] noted the possibility of meibomian gland alternation occurrence within 1 year after the onset of glaucoma eye drops, which requires further study. Consistent with these previous findings, the present study observed a reduction in mean LLT in participants using glaucoma medications, which was characterized mainly by this change in the early 6 months. This is surprisingly in line with the findings of Arita’s study.
In this study, Schirmer test results and conjunctival stain grade reportedly showed improvement in a group of patients using glaucoma medications, which is interpreted as a result of increased tear secretion due to compensation mechanisms caused by deterioration of ocular surface conditions and instability of LLT. Another study by Arita et al. [21] reported that the loss of the meibomian gland increased tear secretion due to the compensatory mechanism.
Although OSDI showed significant deterioration in glaucomatous eyes compared to normal eyes, there was no statistically significant difference in the ocular pain category of the VFQ-25. This may be due to the fact that questions in the VFQ-25 focus on the overall QOL of the patient. Of the 25 questions in the VFQ-25, there is only one question related to ocular surface discomfort.
Similar to previous studies, the present study also observed a reduction in LLT in patients using glaucoma medications. OSDI, which indicates the subjective symptoms of eye dryness, also deteriorated in the group of patients using glaucoma medication. Nevertheless, the total VFQ score in the glaucoma group did not deteriorate compared to that in the control group. Therefore, while glaucoma medication may worsen dry eye syndrome, it does not affect the overall QOL. As glaucoma progression subsequently results in vision loss, the use of glaucoma medications is necessary.
This study observed statistically significant deterioration in scores of near activity and social functioning items, which are related to the QOL, in patients with glaucoma. It is not known whether the scores of these two items deteriorate due to the use of glaucoma medication or the progression of glaucoma. Therefore, further analysis is required.
This study had several limitations. First, the number of participants was relatively small. Therefore, it was difficult to obtain statistically significant results in the subgroup analysis of the group using glaucoma medications. Second, the glaucoma medications analyzed in this study were not studied separately with and without the addition of preservatives. Previous studies have shown that preservation agents such as benzalkonium chloride play a major role in causing dry eye syndrome in patients with glaucoma [1,19,22]. Therefore, the interpretation of our results is limited as it is unclear whether dryness depends on the component characteristics of glaucoma medication or is caused by preservatives such as benzalkonium chloride. Despite these limitations, this study has the strength in that it was prospective. In addition, previous studies have used only objective indicators, such as OSDI, TBUT, and LLT, for ocular surface disease, while the present study assessed the vision-related QOL.
In conclusion, the results of the present study showed a significant reduction in LLT and deterioration of OSDI in patients with glaucoma using glaucoma medications. However, the decrease in LLT only worsened during the initial 6 months and showed no further worsening. Analysis of the VFQ items showed a reduction in near activity and social functioning in patients with glaucoma, even though there was no significant difference between control eyes and glaucomatous eyes in the total VFQ score. These results indicated that there was no significant deterioration in the QOL of patients using glaucoma medication, as represented by the total VFQ score. However, when determining a treatment plan, clinicians should consider treatment compliance, as patients with glaucoma may complain of difficulty in near activity or social functioning.
Notes
No potential conflict of interest relevant to this article was reported.