Progressive Optic Disc Tilt in Young Myopic Glaucomatous Eyes
Article information
Abstract
Purpose
To explore the progressive change and associated factors of optic disc tilt in young myopic glaucomatous eyes by analyzing long-term follow-up data.
Methods
Optic disc images were obtained from spectral-domain optical coherence tomography enhanced depth imaging from at least five different visits. At each visit, the disc tilt angle (DTA), defined as the angle between the Bruch's membrane opening plane and the optic canal plane, was estimated at the central frame that passes through the optic disc. Glaucoma progression was assessed on the basis of changes noted on serial optic disc and retinal nerve fiber layer photographs or changes in the visual field (VF). A linear mixed effect model was used to assess the influence of parameters (age, sex, baseline and follow-up intraocular pressure, retinal nerve fiber layer thickness, VF mean deviation, axial length, central corneal thickness), and presence of glaucomatous progression upon DTA change.
Results
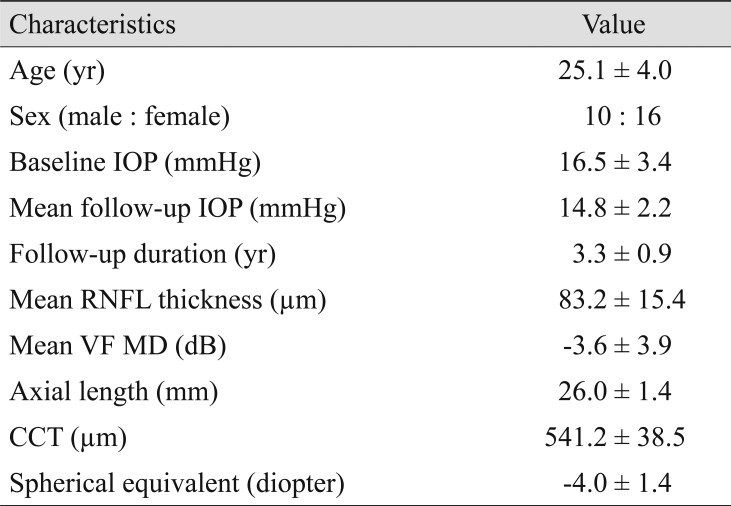
A total of 26 eyes of 26 young myopic primary open-angle glaucoma patients (axial length >24.0 mm; mean age, 25.1 ± 4.0 years; mean follow-up, 3.3 ± 0.9 years) were included. DTA was 7.0 ± 3.4 degrees at baseline and 8.3 ± 3.8 degrees at last visit, which represents a significant difference (p < 0.001). Worse VF mean deviation (p < 0.001) and longer axial length (p = 0.006) were significantly associated with DTA increase.
Conclusions
Young myopic glaucomatous eyes showed progressive optic disc tilting. Progressive optic disc tilting in young myopic glaucomatous eyes may be related to either continuous axial myopic shift or glaucomatous structural change.
Myopia is a risk factor for glaucoma development [123]. Since a considerable portion of glaucoma patients are myopic, the clinical characteristics of myopic glaucoma have become an issue of interest. The reason that myopia draws attention in terms of glaucoma pathogenesis is that myopic eyes usually experience a change in the optic nerve head (ONH) and peripapillary area according to axial elongation. Subsequently, a substantial portion of myopic eyes have a tilted optic disc shape and atrophy in the peripapillary area [4]. During the process of myopic change in the optic disc, the lamina cribrosa (LC) may also undergo deformation because the LC exists within the ONH. A recent study revealed that eyes with a longer axial length (AXL) had a thinner LC [5], and those LC changes may affect glaucoma progression [678]. As demonstrated in previous studies, the LC of the ONH is known as the primary site of glaucoma pathogenesis [91011].
Hence, we hypothesized that, if the ONH would change configuration over time in myopic eyes, this change may have some relationship with glaucomatous structural impairment. Therefore, in the present study, we explored the progressive change of the optic disc tilt in myopic glaucomatous eyes by analyzing longitudinal follow-up data. With the results of the current study, we expected to elucidate the relationship between the progressive change of ONH configuration and glaucoma progression in myopic glaucomatous eyes. In this study, we included young myopic glaucomatous eyes in particular since the LC and/or ONH of younger patients are more flexible or malleable than those of older patients.
Materials and Methods
Patients
The data were collected from an ongoing Asan Glaucoma Progression Study. Between January 2011 and March 2017, the medical records of all patients who were evaluated by a single glaucoma specialist (KRS) at the glaucoma clinic of Asan Medical Center, Seoul, Korea were retrospectively reviewed in this longitudinal, observational study.
Initial testing included a comprehensive ophthalmologic examination, which included a medical history review, best-corrected visual acuity measurement, slit-lamp biomicroscopy, multiple intraocular pressure (IOP) measurements using Goldmann applanation tonometry, gonioscopy, dilated fundoscopic examination using a 90- or 78-diopter lens, stereoscopic optic-disc photography, retinal nerve fiber layer (RNFL) photography, visual field (VF) testing, central corneal thickness measurement (DGH-550 instrument, DGH Technology, Exton, PA, USA), and AXL measurement (IOL Master, Carl Zeiss Meditec, Dublin, CA, USA). RNFL thickness measurement was performed using spectral-domain optical coherence tomography (SD-OCT; Spectralis OCT, Heidelberg Engineering, Dossenheim, Germany). Young glaucoma patients aged 17 to 30 years old at baseline were selected. The inclusion criteria at the initial assessment included a best-corrected visual acuity of 20 / 40 or better, an AXL ≥24 mm, a normal anterior chamber, an open angle on slit-lamp and gonioscopic examinations, and all IOP measurements lower than 21 mmHg. Patients with glaucomatous optic disc changes, such as diffuse or focal neural rim thinning, disc hemorrhage, or RNFL defects, as confirmed by two glaucoma specialists (KRS and JWS), were included. Patients with any other ophthalmic or neurologic condition that could result in a VF defect, a history of diabetes mellitus, or severe myopic fundus changes precluding adequate examinations were excluded. Pseudophakic and aphakic eyes were also excluded. If both eyes in the same patient were found to be eligible, one eye was randomly selected for analysis.
All patients were followed up at 6-month intervals for at least 2 years using stereoscopic optic disc/RNFL photography, VF testing, and SD-OCT scanning. All tests were performed at the same visit or within 2 weeks thereof. Furthermore, at least five qualified SD-OCT images obtained at different visits were required for the patient to be included in the study. If the patient underwent intraocular surgery or laser therapy during the follow-up period, only data obtained before such operations, which should have been longer than 2 years prior to the operation, were included.
VF tests were performed using a Humphrey field analyzer (Swedish interactive threshold algorithm 24-2, Carl Zeiss Meditec). Only reliable VF test results (false-positive errors <15%, false-negative errors <15%, and fixation loss <20%) were included. The VF test was repeated within 2 weeks of the baseline measurement for confirmation. Patients were expected to return approximately 1 month after the baseline examination in order to assess their responses to medication and undergo a third VF test. Hence, all patients underwent three VF tests within the first 6 weeks. Data from the first VF test were excluded to obviate any learning effects; and the results of the second and third VF tests, which were performed within 1 month of each other, were considered the baseline examinations. Patients who underwent ≥5 reliable VF tests, excluding the first VF test, were included. Glaucomatous VF defects were defined by glaucoma hemifield test results outside the normal limits or a pattern standard deviation outside the 95% range of normal limits. In addition, these eyes had to have a cluster of 3 points with probabilities <5% on the pattern deviation map in ≥1 hemifield, including ≥1 point with a probability <1% or a cluster of 2 points with a probability <1%. Glaucomatous VF defects had to be confirmed on ≥2 VF examinations. All patients underwent medical therapy. The study was approved by the institutional review board of Asan Medical Center (2017-1311), and the study design followed the principles of the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of this study.
Measurement of the optic disc tilt angle
Spectral-domain enhanced depth imaging was used to scan the optic disc. Brief ly, the entire optic disc was scanned using a 6-mm length line (512 A-scans) with an interval of 50 µm. In our study, an average of 35 horizontal B-scans was produced in enhanced depth imaging mode. From these B-scans, the frame that passed through the optic disc center was selected. The structure of the optic disc was analyzed with the intrinsic viewer. Images were imported to ImageJ software ver. 1.48 (National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij) and magnified by two. Bruch's membrane opening (BMO, points A and B in Fig. 1) was defined by the proximal tips of the Bruch's membrane. A line connecting the BMO on each side (line AB) was regarded as the BMO plane.

Measurement of lamina cribrosa depth and disc tilt angle. Bruch's membrane opening (BMO, points A and B) was defined by the proximal tips of the Bruch's membrane. A line connecting the BMO on each side (line AB), was regarded as the BMO plane. The disc tilt angle was defined as the angle between the BMO plane and the optic canal plane (line AC) that passes through the optic disc (θ).
A line (line AC) connecting the nasal BMO and the proximal margin of the external oblique border tissue was drawn and set as the optic canal plane. The disc tilt angle (DTA) was defined as the angle between the BMO plane and the optic canal plane that passes through the optic disc (θ). The DTA was measured using the ‘angle tool’ in the ImageJ software. All accepted images exhibited a centered optic disc, were well-focused, and had even and adequate illumination. Eyes were excluded when these points could not be clearly identified. All measurements were performed by a single well-trained examiner (JYY). Before the main analysis, an intra-examiner intra-class correlation coefficient was calculated using 20 randomly selected images to test the reproducibility of the DTA measurements. Intra-examiner intraclass correlation coefficient value for DTA was 0.947 (95% confidence interval, 0.866 to 0.983). Inter-examiner intraclass correlation coefficient value was 0.909 (95% confidence interval, 0.817 to 0.975).
Assessment of glaucomatous progression
Glaucoma progression was determined by either structural or functional aspects. Structural progression was assessed by serial optic disc/RNFL photographs. Two glaucoma experts (KRS and JWS) independently assessed all photographs to estimate glaucoma progression. In each patient, the most recent photograph was compared with the baseline photograph. The two graders were unaware of the progression assessments made by the other, and each grader viewed all photographs of each eye before making an assessment. Both graders were asked to determine glaucomatous optic disc or RNFL progression as demonstrated by the increase in the extent of neuroretinal rim thinning, enhancement of disc excavation and/or any widening, deepening, or new RNFL defects. The graders classified each glaucomatous eye as either stable or progressing and determined the type of structural change. Subjects were classified with progression when an eye showed progression on either the optic disc or RNFL photograph. If the RNFL photographs were difficult to evaluate because of diffuse atrophy or invisible RNFL layers due to a lightly pigmented fundus, progression was determined using optic disc assessment. If the opinions of the two observers differed, a consensus was reached after discussion.
VF progression was determined by the Early Manifest Glaucoma Trial criteria or the linear regression analysis of mean deviation (MD) values. Definite Early Manifest Glaucoma Trial progression required that at least three test points were flagged as significantly deteriorated at the same test point locations in 3 consecutive fields [12]. These changes should also have been observed at the latest follow-up visit. In the linear regression, VF progression was defined as a significant negative slope between MD and age.
Statistical analysis
DTA measurements at baseline and last follow-up were compared using the Wilcoxon signed rank test. Demographics and clinical characteristics were compared between progressed eyes and stable eyes in terms of glaucomatous damage using the Mann-Whitney U test. A linear mixed effect model was used to assess the influence of parameters (age, sex, baseline IOP, mean follow-up IOP, RNFL thickness, VF MD, AXL, central corneal thickness, and presence of glaucomatous progression) upon the DTA change. Variables with a p-value ≤0.2 in univariate analyses were included in a multiple mixed effects model. The final models for outcomes were determined by backward elimination procedures. A p-value less than 0.05 was considered statistically significant. R ver. 3.2.2 (R Core Team, 2013) and IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY, USA) were used for the statistical analyses.
Results
A total of 26 eyes from 26 participants were enrolled in this study. Out of the 26 participants, 10 were men and 16 were women. Eleven eyes had a glaucomatous VF defect on repeated baseline examinations, while the remaining 15 eyes had a glaucomatous optic disc without apparent VF defect. The average age at baseline was 25.1 years old, with a 95% confidence interval of 22.4 to 26 years old. The mean follow-up duration for all subjects was 3.3 years. The participants' characteristics are described in Table 1. The mean DTA was significantly greater at the last visit compared to baseline (7.0 ± 3.4 vs. 8.3 ± 3.8 degrees, p < 0.001). The mean DTA continuously increased over time (Fig. 2).

Disc tilt angle change over time in all eyes. Redline indicates eyes which show glaucomatous progression while blue line indicates stable eyes.
Among 26 eyes, six eyes showed glaucomatous progression. When compared with stable eyes, progressed eyes showed worse baseline glaucoma severity as determined by VF MD (median [range], −6.6 dB [−15.3 to −0.49] vs. −1.9 dB [−8.4 to −0.1], p = 0.047) and SD OCT-measured RNFL thickness (70.8 micron [51.5 to 80.3] vs. 88.1 micron [58.8 to 110.3], p = 0.028). No other parameters were significantly different (Table 2).
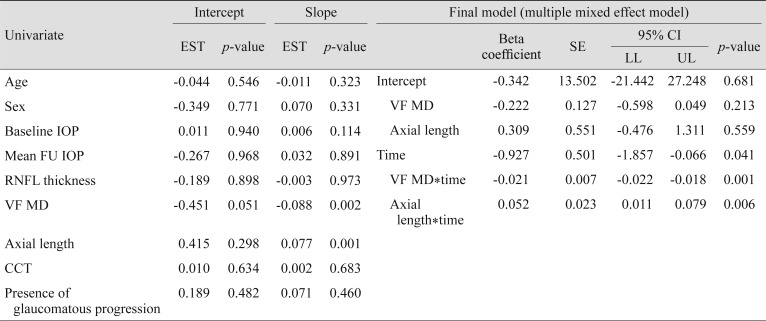
When we assessed the factors associated with a progressive increase of DTA, worse VF MD (p < 0.001) and longer AXL (p = 0.006) were significant associations (Table 3).
Clinical examples are shown in Fig. 3 and 4. A 28-year-old woman had progressive tilting in her optic disc (baseline, 9.3 degrees; 39 months later, 10.4 degrees) during the follow-up period (Fig. 3A, 3B). Her VFs and RNFL photographs showed rapid progression (Fig. 3C). A 30-year-old man (AXL, 29.2 mm) showed progressive tilting in his optic disc (Fig. 4A) and SD-OCT imaging (baseline, 8.6 degrees; 52 months later, 11.7 degrees) (Fig. 4B). During the follow-up period, however, there was no evidence of glaucomatous progression either in the structural or functional test (Fig. 4C).

Representative case example is shown. Upper images were obtained at baseline examination, and lower images were obtained 39 months later from baseline. (A,B) A 28-year-old woman had progressive tilting in her optic disc (baseline, 9.3 degrees; 39 months later, 10.4 degrees) and deepening of the lamina cribrosa (baseline, 359.9 microns; 39 months later, 366.9 microns). (C) Her visual field and retinal nerve fiber layer photographs showed rapid progression.

Representative case example is shown. Upper images were obtained at baseline examination and lower images were obtained 52 months later from baseline. (A,B) A 30-year-old man showed progressive tilting in his optic disc (baseline, 8.6 degrees; 52 months later, 11.7 degrees and deepening of lamina cribrosa baseline, 351.5 micron; 52 months later, 380.7 micron), but (C) there was no evidence of glaucomatous progression either in the structural or functional test.
Discussion
In our cohort, young myopic glaucoma patients with a mean age of 25.1 years were followed for about 3.3 years. Approximately 23% of the eyes showed glaucomatous progression during the follow-up period. Progressed eyes showed significantly thinner RNFL and worse baseline VF compared with stable eyes. This observation was in line with our previous study performed with young myopic glaucomatous eyes [1314].
Interestingly, DTA measured at the central plane significantly increased during the follow-up period. Hence, young myopic glaucomatous eyes seem to experience significant changes in terms of optic disc configuration. The observation of an increase in DTA reflected that the optic disc underwent progressive tilting over time in some young myopic glaucomatous eyes. Since optic disc tilting is known to be caused by myopic expansion in peripapillary sclera according to the axial elongation [1516], this may suggest that there would be continuous myopic changes in the optic disc of some young myopic glaucomatous eyes. When we explored the factors associated with the increase of DTA, longer AXL was a significant factor. Hence, high myopic glaucomatous eyes with longer AXL tended to experience progressive optic disc tilting in adolescence. Along with a longer AXL, worse baseline VF MD showed an association with progressive optic disc tilting. A possible explanation for this could be that glaucomatous damage, like pressure on the optic disc, aggravates tilting by weakening the peripapillary area, which is vulnerable to mechanical pressure [41718]. However, this is a speculation that warrants further investigation.
Although progressive optic disc tilt was associated with worse baseline glaucoma severity determined by VF MD, progressive optic disc tilt did not reveal a direct relationship with glaucomatous progression. Optic disc tilt may strain the RNFL which leads to injury; this could result in glaucoma-like structural damage [15]. However, as shown in Fig. 3 and 4, some of the eyes with progressive optic disc tilt revealed rapid glaucomatous progression while others were stable in terms of glaucomatous change during the follow-up period. Some publications reported that a tilted optic disc was a positive prognostic factor for glaucoma progression [192021]. In our study, prognosis of progressive optic disc tilt in terms of glaucomatous change was variable among the participants. However, we could confirm that progressive optic disc tilt and longer AXL have some level of relationship with glaucomatous change.
Our current study had several notable limitations. First, the relatively small sample size should be considered in interpreting our results. Also, the DTA was measured at the central frame only. In myopic optic discs, DTA measurement at other frames was not reproducible due to irregularly-shaped optic disc configuration; therefore we focused on the central frame which was consistently accessible at each follow-up. Additionally, long-term AXL data were not available and were excluded. Correlation between longitudinal AXL change and DTA increment should be investigated in the forthcoming study.
In conclusion, young myopic glaucomatous eyes showed progressive tilting in their optic disc shape. A previous publication reported that myopic eyes underwent progressive tilting and increased peripapillary atrophy in the childhood period [1516]. According to our results, some myopic glaucomatous eyes experienced continuous titling in their optic discs even in adolescence. Both longer AXL and glaucomatous change contributed to this optic disc tilt. Progressive optic disc tilting may affect LC configuration; thus, progressive optic disc tilting in young myopic glaucomatous eyes may be related to either continuous myopic optic disc shift according to axial elongation or glaucomatous structural damage to the optic disc and peripapillary sclera.
Acknowledgements
This study was supported by a grant (2018-0303) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, South Korea, and by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science, and Technology (NRF-2017R1A2B4007792).
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.