 |
 |
| Korean J Ophthalmol > Volume 33(1); 2019 > Article |
Abstract
Purpose
To investigate the risk factors of diabetic nephropathy in patients with diabetic retinopathy requiring panretinal photocoagulation (PRP) and the visual prognosis.
Methods
A retrospective review of electronic medical records was conducted at Seoul St. Mary's Hospital, comprising 103 patients with type 2 diabetes mellitus and diabetic retinopathy who underwent PRP from 1996 to 2005. Patients with type 1 diabetes mellitus, non-diabetic renal disease, non-diabetic retinal disease, visually significant ocular disease, high-risk proliferative diabetic retinopathy, and advanced diabetic retinopathy were excluded. The patients were divided into three groups: no nephropathy (group 1, n = 45), microalbuminuria (group 2, n = 16), and advanced nephropathy (group 3, n = 42). Duration of diagnosis of retinopathy and nephropathy, glycosylated hemoglobin, visual acuity, complications, and treatment history were investigated.
Results
The mean glycosylated hemoglobin of group 3 (8.4 ± 1.2) was higher than that of group 1 (7.7 ± 1.0) or group 2 (7.7 ± 1.0) (p = 0.04). Mean interval from PRP to diagnosis of nephropathy was 8.8 ± 6.0 years in group 2 and 8.7 ± 4.9 years in group 3. The significant decrease in visual acuity in group 3 (28 eyes, 35.9%) was significantly higher than that in group 1 (15 eyes, 18.1%, p = 0.01) or group 2 (6 eyes, 20.7%, p = 0.03). Only vitreous hemorrhage showed a significantly higher incidence in groups 2 and 3 than in group 1 (p = 0.02). Multivariate regression analysis revealed that female sex and lower glycosylated hemoglobin were significantly associated with a protective effect on development of nephropathy.
Diabetes mellitus (DM) is a chronic disease requiring continuous care and management. Complete cure is exceedingly rare. One of the microvascular complications of DM, diabetic retinopathy, is a major cause of visual loss [1]. About 4% of patients with type 1 DM and 1.6% of patients with type 2 DM lose their vision due to progression of diabetic retinopathy. Although the rate of blindness in patients with type 2 DM is lower than that of type 1 DM, more patients with type 2 DM suffer from diabetic retinopathy because of its higher incidence compared with type 1 DM [2].
Diabetic nephropathy is also a major microvascular complication of DM. In the United States, the incidence of diabetic nephropathy is 20% to 30% in patients with type 1 DM and <20% in patients with type 2 DM. Although patients with type 2 DM show a lower incidence of diabetic nephropathy, about 60% of diabetic end-stage renal disease patients have type 2 DM because of the higher incidence of type 2 DM [3].
Panretinal photocoagulation (PRP) is a major treatment of severe diabetic retinopathy and reduces the risk of severe visual loss by 50% or more, especially for patients with high-risk proliferative diabetic retinopathy (neovascularization with pre-retinal or vitreous hemorrhage, neovascularization at disc) [4,5]. PRP-requiring diabetic retinopathy could be a possible indicator of progression of diabetic microvascular complications.
Diabetic retinopathy, especially at the proliferative stage, could be a useful tool for diagnosis and screening of diabetic nephropathy [6]. Also, the presence and severity of diabetic retinopathy are closely associated with markers of diabetic kidney disease, especially lower estimated glomerular filtration [7]. The Korea National Health and Nutrition Examination Survey of the Korean population revealed the association of diabetic retinopathy and diabetic nephropathy [8]. However, the cross-sectional design hindered the understanding of the natural history of diabetic retinopathy and diabetic nephropathy.
The aims of this study were to investigate the incidence, time to diagnosis of diabetic retinopathy, and risk factors of diabetic nephropathy in patients with PRP-requiring diabetic retinopathy and to explore an association among diabetic nephropathy, retinal complications, and visual prognosis.
This study was performed with approval of the institutional review board of Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea (KC16RISI0402), and was conducted in adherence with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all patients.
Designed as a retrospective single center study, electronic medical records of patients with type 2 DM were reviewed. Patients diagnosed with diabetic retinopathy who underwent PRP from 1996 to 2005 were included. Patients with type 1 DM, non-diabetic renal disease, non-diabetic retinal disease, visually significant ocular disease in both eyes except cataract, or more than a year of follow-up loss period were excluded. Also, patients diagnosed with advanced diabetic retinopathy with complications requiring immediate surgical treatment [5,9], such as vitreous hemorrhage or tractional retinal detachment at the first ophthalmologic visit, were excluded because of the absence of regular ophthalmologic check-ups and their far advanced disease stage compared with other patients with PRP-requiring diabetic retinopathy patients.
All patients were diagnosed with DM according to the criteria in the Expert Committee on Diagnosis and Classification of Diabetes Mellitus [10,11]. Treatment comprised either hypoglycemic agents or insulin therapy after their confirmed diagnosis of diabetes. Patients regularly followed up with endocrinologists; follow-up procedures included annual checks of blood chemistry, urine chemistry, and glycosylated hemoglobin. Also, the patients had regular funduscopic exams by retina specialists without follow-up loss from first diagnosis of diabetes to 2015.
For evaluation of diabetic retinopathy, patients reported to the ophthalmology department and underwent comprehensive ophthalmologic examinations including fundus exam with a wide-field contact lens, fundus photography, and optical coherence tomography if needed. The stage of diabetic retinopathy was determined by comparison with standard photographs from the Early Treatment Diabetic Retinopathy Study (ETDRS) [12]. If proliferative diabetic retinopathy or progression of severe non-proliferative diabetic retinopathy was suspected in the fundus photography, fluorescein angiography was conducted. Indications of PRP were defined as proliferative diabetic retinopathy, very severe non-proliferative diabetic retinopathy [12], or aggravation of severe non-proliferative diabetic retinopathy.
Any visual threatening complications of diabetic retinopathy, including vitreous hemorrhage, macular edema, tractional retinal detachment, or neovascular glaucoma, were treated appropriately by periocular or intravitreal injection or steroid or anti-vascular endothelial growth factor (VEGF) agents, pars plana vitrectomy, intraocular pressure lowering agents, or glaucoma surgery. For the patients with a visually significant cataract and significant decrease of visual acuity (over 2 lines in the Snellen chart) that could not be explained by significant retinal complications or with blurred fundus imaging, cataract surgery was performed.
Included patients were divided into three groups according to nephropathy status. Patients without diabetic nephropathy who showed no albuminuria and a normal glomerular filtration rate were classified as group 1 (no nephropathy group). Patients with microalbuminuria (microalbuminuria group) were classified as group 2. Patients with overt proteinuria, chronic kidney disease, or endstage renal disease (advanced nephropathy group) were classified as group 3. Microalbuminuria was defined as urine albumin excretion between 30 and 299 µg in a 24-hour collection of urine, between 20 and 199 µg/min in a timed urine collection, or between 30 and 299 µg/g of spot urine albumin/creatinine ratio [13].
By retrospective clinical chart review, we investigated mean age, sex ratio, duration of DM at diagnosis of diabetic retinopathy and nephropathy, mean glycosylated hemoglobin (HbA1c), and morbidity of hypertension (repeatedly measured systolic blood pressure over 140 mmHg or diastolic blood pressure over 90 mmHg in a resting state, excluding patients with prophylactic prescription of angiotensin converting enzyme inhibitor, beta blocker, or other anti-hypertensive agents). These measurements were clinical signs predicting diabetic nephropathy in diabetic retinopathy patients. Mean age, mean HbA1c, and morbidity of hypertension were investigated at the time of diagnosis of diabetes, retinopathy, and nephropathy, respectively. Additionally, we investigated the secondary outcome measures, visual acuity at the time of PRP and last follow-up, ocular complications (macular edema, vitreous hemorrhage, tractional retinal detachment, neovascular glaucoma), and treatment history. If a patient had a non-diabetic retinal disease, corneal opacity, or advanced glaucoma not associated with complications of diabetes in a single eye, then data of visual acuity, ocular complications, and treatment history were investigated for only the other eye. Collected data were analyzed and compared by group.
The statistical analysis was performed with IBM SPSS Statistics ver. 19.0 (IBM Corp., Armonk, NY, USA). A p-value < 0.05 was defined as statistical significance.
The 103 patients were divided into group 1 (n = 45, 84 eyes), group 2 (n = 16, 29 eyes), and group 3 (n = 42, 78 eyes). There were no statistically significant differences among the groups in mean age at diagnosis of diabetes (44.7 ± 9.2, 41.4 ± 10.7, 45.3 ± 8.6 years, respectively) or mean age at the time of PRP (56.6 ± 8.4, 52.3 ± 10.5, 58.6 ± 9.3 years, respectively) by ANOVA test (p = 0.349 and p = 0.063, respectively). Also, the mean age at diagnosis of diabetes nephropathy in group 2 (61.0 ± 11.7 years) and group 3 (67.3 ± 9.4 years) was not statistically different (p = 0.067, Mann-Whitney U-test). There were no statistically significant differences in age among groups 1, 2, and 3. The proportions of males in groups 1, 2, and 3 were statistically significantly different (9 out of 45, 5 out of 16, 13 out of 42, respectively) by the Kruskal-Wallis test (p = 0.001). Group 1 showed a lower proportion of males than groups 2 and 3 (p < 0.001 and p < 0.001, respectively, Mann-Whitney U-test).
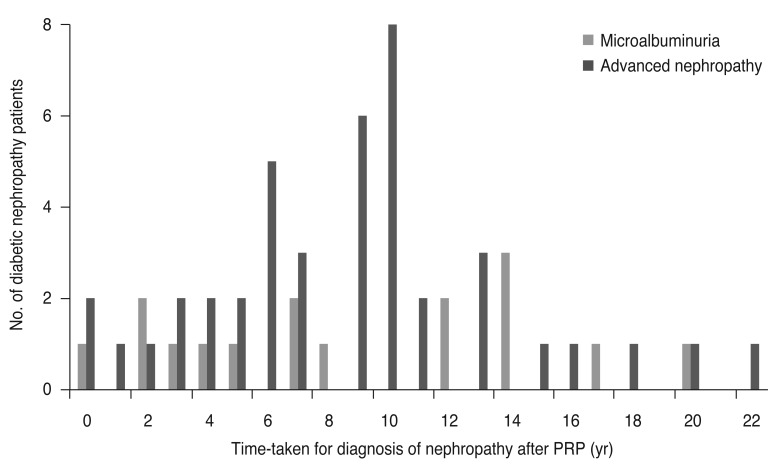
Mean duration of diabetes (24.7 ± 4.7, 24.1 ± 4.4, 25.9 ± 5.0 years, respectively), mean follow-up period after PRP (12.8 ± 4.9, 13.3 ± 5.0, 12.6 ± 4.6 years, respectively), and mean duration of DM at PRP (11.9 ± 6.0, 10.8 ± 4.8, 13.3 ± 5.1 years, respectively) were similar among the three groups (p = 0.248, p = 0.846, and p = 0.222 respectively, Kruskal-Wallis test) (Table 1). Mean intervals from the time of PRP to diagnosis of nephropathy in groups 2 (8.8 ± 6.0 years) and 3 (8.7 ± 4.9 years) were not significantly different (p = 0.972, Mann-Whitney U-test). One patient in group 2 and three patients in group 3 were diagnosed with diabetic nephropathy at or before PRP. Of 58 patients with diabetic nephropathy, 27 were diagnosed with nephropathy 6 to 10 years after PRP (Fig. 1). In groups 1, 2, and 3, mean HbA1c at DM diagnosis (7.73 ± 1.0, 7.70 ± 1.0, 8.37 ±1.2, respectively) and mean HbA1c at diabetic retinopathy diagnosis (7.6 ± 1.3, 9.8 ± 2.5, 8.1 ± 2.0, respectively) were statistically difference (p = 0.04, p = 0.008, respectively, ANOVA test). However, mean HbA1c at the time of diabetic nephropathy diagnosis in groups 2 and 3 (9.6 ± 1.8, 8.2 ± 1.3, respectively) was not statistically different (p = 0.128, Mann-Whitney U-test). Proportions of patients with hypertension (35.5%, 31.3%, 33.3%, respectively) showed no statistical difference (p = 0.947, Kruskal-Wallis test). Mean visual acuity at PRP and last follow-up did not differ among the three groups (p > 0.05 for all, Kruskal-Wallis test) (Table 2).
Of the group 3 eyes, 35.9% (28 of 78 eyes) showed a significant decrease in visual acuity, which was significantly higher than the 18.1% (15 of 83 eyes) of group 1 and 20.7% (6 of 29 eyes) of group 2 (p = 0.029). Ocular complication rates except vitreous hemorrhage and number of treatments did not differ among the three groups (p > 0.05 for all). Only vitreous hemorrhage showed a significantly higher incidence in groups 2 (7 eyes, 24.1%) and 3 (15 eyes, 19.2%) than in group 1 (3 eyes, 3.6%) (p = 0.017, Kruskal-Wallis test). The rate of significant visual loss (more than two lines in the Snellen chart) showed a statistical difference among the three groups (p = 0.02, Kruskal-Wallis test). More cases of pars plana vitrectomy were found in groups 2 and 3 than in group 1, though the difference was not significant (p = 0.265, Kruskal-Wallis test) (Table 3).
Multivariate regression analysis determined that female sex and lower mean HbA1c had a protective effect on development of diabetic nephropathy (p < 0.001, p = 0.043, respectively). Duration of diabetes, duration of treatment for retinopathy, and hypertension at the time of DM and diabetic nephropathy diagnoses did not significantly influence development of diabetic nephropathy (p = 0.644, p = 0.742, and p = 0.484, respectively) (Table 4).
We investigated the natural history of diabetic microvascular complications. In patients with PRP-requiring diabetic retinopathy, many developed diabetic nephropathy an average of 8 to 9 years later than in those without PRP. Several risk factors have been found to influence susceptibility to the microvascular complications of DM. DM duration, poor glycemic control, and arterial hypertension have consistently been shown to be correlated with diabetic retinopathy and diabetic nephropathy. In our study, male sex and poor control of blood glucose (higher HbA1c) were statistically significant for diabetic nephropathy development. A number of studies has demonstrated that level of HbA1c, diabetes duration, and high blood pressure are important risk factors for diabetic nephropathy [14]. However, in our study, high blood pressure and duration of diabetes were not statistically significant.
The natural history of microvascular complications of type 1 diabetes, retinopathy, and nephropathy are well-known because of their relatively precise onset time compared with type 2 DM. For patients with type 1 DM, the incidence of diabetic retinopathy (prevalence of 50% at about 7 years of treatment) seems to rise earlier than that of diabetic nephropathy (first incidence peak at 16 years) [15,16]. In addition, it is difficult to know the exact onset time of type 2 DM compared with type 1 DM. Patients first diagnosed with type 2 DM could have any stage of microvascular complications, retinopathy, and nephropathy. Though inclusion criteria were very limited, the results of this study partially show the clinical history of microvascular complications of diabetes.
Development of nephropathy and its severity showed a significant relationship with more frequent retinal complications and poor visual outcomes. Patients with nephropathy in this study showed a higher rate of compromised visual acuity (20.7% and 35.9% in groups 2 and 3, respectively) than the no-nephropathy group 1 (18.1%) and a higher incidence of vitreous hemorrhage (40% and 36% in groups 2 and 3, respectively) than the no-nephropathy group (5%). It is difficult to explain why the severe form of diabetic retinopathy occurred.
As a retrospective observational study, this study has some limitations, like uncontrolled demographic factors and limited number (103) of subjects. There could be a bias in the included patients because they all underwent regular retinopathy and nephropathy follow-up, and patients with poor compliance as well as those without retinopathy were excluded. In the real world, development and progression of nephropathy could be faster and more severe, and the relationship between nephropathy and ocular complications or its treatment could differ from the results of this study [17].
Because this study included long-term follow-up, a maximum 20-year follow-up period, there were changes in the diagnostic criteria of diabetes (e.g., HbA1c level) [18] and treatment tools (e.g., intravitreal injection of anti-VEGF agents) over time [19]. Development of an intravitreal injection treatment including anti-VEGF agents changed the treatment modality for diabetic retinopathy [19]. Changes in diagnosis criteria and treatment modality could be considerable confounding factors when interpreting the results of this study. Cataract, which was not fully controlled in this study, also could be a confounding factor for visual acuity. To control these factors, well controlled future studies with consistent diagnosis and treatment criteria should be conducted for investigation of the natural history of type 2 DM complications.
It has not been long since clinicians realized the importance of microalbuminuria for the study of diabetic nephropathy [20]. Since the period of inclusion and follow-up was about 20 years, the detailed diagnostic or screening criteria were changed during the study [20,21]. It is possible that the change in diagnostic criteria for diabetic nephropathy played a role as a confounding factor [22]. The change in criteria made it difficult to evaluate the progression of diabetic nephropathy, and a smaller portion of microalbuminuria compared with advanced nephropathy could have been affected by the change. Further research is needed on the natural history of diabetic nephropathy.
It was accepted previously that development of retinopathy preceded a development of nephropathy in the majority of cases [23,24]. Diabetic retinopathy was a possible clinical marker for diabetic nephropathy [6,7]. Recently, there were some reports introducing patients with diabetic nephropathy without retinopathy [25,26], and there were 2 patients with simultaneous diagnosis of diabetic retinopathy and nephropathy in this study. Because this study excluded patients without diabetic retinopathy, it does not address the clinical findings of nephropathy without diabetic retinopathy.
Cortes and Mogensen [27] showed genetic differences affecting the susceptibility to damage of each system for a given level of exposure to high blood pressure, hyperglycemia, and other risk factors. Inflammation, oxidative stress, and coagulability may also have effects on both retinopathy and nephropathy complications. Identification of genetic polymorphisms associated with risk for one or both complications may lead to newer preventive treatments in diabetic persons at risk of developing the earlier preclinical stages of the disease [28]. A lack of consideration of genetic differences could be one of the limitations of this study. To investigate the natural history of microvascular complications of type 2 diabetes, a field study design with wider inclusion criteria, more subjects, and control of confounding factors will be necessary.
Despite these limitations, this study showed that many patients with PRP-requiring diabetic retinopathy developed diabetic nephropathy during long-term follow-up. Higher HbA1c was the most significant feature of advanced nephropathy, which may imply an uncontrolled blood sugar level in patients. Because development of diabetic nephropathy is associated with higher incidence of ocular complications, ophthalmologists should carefully consider control of blood glucose and development of diabetic nephropathy. Consultation with a physician should not be delayed, especially in male patients with high HbA1c and a long-term follow-up period after PRP.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A1A03010528).
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA 2003;290:2057-2060.


2. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1984;102:520-526.


3. Skyler JS. Microvascular complications: retinopathy and nephropathy. Endocrinol Metab Clin North Am 2001;30:833-856.


4. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology 1981;88:583-600.


5. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98:786-806.


6. He F, Xia X, Wu XF, et al. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia 2013;56:457-466.


7. Man RE, Sasongko MB, Wang JJ, et al. The association of estimated glomerular filtration rate with diabetic retinopathy and macular edema. Invest Ophthalmol Vis Sci 2015;56:4810-4816.


8. Lee WJ, Sobrin L, Lee MJ, et al. The relationship between diabetic retinopathy and diabetic nephropathy in a population-based study in Korea (KNHANES V-2, 3). Invest Ophthalmol Vis Sci 2014;55:6547-6553.


9. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology 1991;98:741-756.


10. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183-1197.



11. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5-S20.



12. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98(5 Suppl):807-822.


13. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005;28:164-176.


14. Kohner EM, Stratton IM, Aldington SJ, et al. Microaneurysms in the development of diabetic retinopathy (UKPDS42). UK Prospective Diabetes Study Group. Diabetologia 1999;42:1107-1112.


15. Palmberg P, Smith M, Waltman S, et al. The natural history of retinopathy in insulin-dependent juvenile-onset diabetes. Ophthalmology 1981;88:613-618.


16. Andersen AR, Christiansen JS, Andersen JK, et al. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 1983;25:496-501.


17. Hartnett ME, Key IJ, Loyacano NM, et al. Perceived barriers to diabetic eye care: qualitative study of patients and physicians. Arch Ophthalmol 2005;123:387-391.


18. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327-1334.



19. Wykoff CC. Impact of intravitreal pharmacotherapies including antivascular endothelial growth factor and corticosteroid agents on diabetic retinopathy. Curr Opin Ophthalmol 2017;28:213-218.


20. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225-232.


21. Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care 2004;27(Suppl 1):S79-S83.


22. Liew BS, Perry C, Boulton-Jones JM, et al. Diabetic nephropathy: an observational study on patients attending a joint diabetes renal clinic. QJM 1997;90:353-358.


23. Lee WJ, Sobrin L, Kang MH, et al. Ischemic diabetic retinopathy as a possible prognostic factor for chronic kidney disease progression. Eye (Lond) 2014;28:1119-1125.



24. Jeng CJ, Hsieh YT, Yang CM, et al. Diabetic retinopathy in patients with diabetic nephropathy: development and progression. PLoS One 2016;11:e0161897.



25. Takao T, Matsuyama Y, Yanagisawa H, et al. Visit-to-visit variability in systolic blood pressure predicts development and progression of diabetic nephropathy, but not retinopathy, in patients with type 2 diabetes. J Diabetes Complications 2014;28:185-190.


26. Ciray H, Aksoy AH, Ulu N, et al. Nephropathy, but not angiographically proven retinopathy, is associated with neutrophil to lymphocyte ratio in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2015;123:267-271.


27. Cortes P, Mogensen CE. The diabetic kidney. Totowa: Humana Press; 2006. p. 473-498.
Fig. 1
Distribution of patients by time-taken for diagnosis of nephropathy after panretinal photocoagulation (PRP). In the microalbuminuria group and advanced nephropathy group, one and three patients, respectively, were diagnosed as diabetic nephropathy at or before the time of PRP. In 58 patients with diabetic nephropathy, 27 were diagnosed nephropathy 6 to 10 years after PRP.
- TOOLS
-
METRICS

-
- 11 Crossref
- 0 Scopus
- 3,724 View
- 39 Download
- Related articles
-
Analgesic Effects of Tramadol During Panretinal Photocoagulation2009 December;23(4)





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



