Effect of Preoperative Factor on Intraocular Pressure after Phacoemulsification in Primary Open-angle Glaucoma and Primary Angle-closure Glaucoma
Article information
Abstract
Purpose
To compare the effects of cataract surgery on intraocular pressure (IOP) according to preoperative factor in primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG).
Methods
The medical records of 75 POAG and 95 PACG patients who underwent cataract surgery were reviewed. We classified POAG patients with a preoperative peak IOP of less than 31 mmHg and less than three medications used before surgery and PACG patients with a peak IOP of less than 42 mmHg, less than three medications used, and peripheral anterior synechiae of less than four clock hours into group 1. Patients with levels exceeding these thresholds were classified into group 2. The IOP, numbers of medications, and success rates were compared between two groups.
Results
At 36 months after surgery, IOP reduction in group 1 was significantly greater than that in group 2 among POAG patients (−1.7 ± 2.1 vs. −0.6 ± 2.0 mmHg, p = 0.021); however, there was no significant difference between the two groups for PACG patients (−2.5 ± 2.0 vs. −2.2 ± 3.3 mmHg, p = 0.755). The medication changes were similar between the two groups for both POAG and PACG patients. The success rate at 36 months was significantly higher in group 1 than in group 2 for POAG patients (66.7% vs. 35.7%, p = 0.009), but there was no significant difference between the two groups for PACG patients (79.1% vs. 69.2%, p = 0.264).
Conclusions
For patients with relatively low peak IOP who used fewer medications before surgery, cataract surgery alone was effective for IOP control in both POAG and PACG patients. Conversely, For POAG patients with a history of higher peak IOP and who used more medications, cataract surgery was not effective in lowering IOP, whereas it resulted in relatively good IOP values in PACG patients.
As the average life expectancy increases, the number of patients with both glaucoma and cataract is also rising. If a patient has medically uncontrolled glaucoma and coexisting visually significant cataract, it can be difficult to determine the appropriate surgical management. In this situation, the surgeon can choose one of the following surgical procedures: cataract surgery alone followed by medical antiglaucoma treatment or glaucoma surgery, glaucoma surgery alone followed by later cataract surgery, or combined cataract and glaucoma surgery.
When glaucoma filtering surgery is performed first, the cataract may progress more rapidly despite a significant improvement in the intraocular pressure (IOP) [123]. Subsequent cataract surgery may affect the function of the existing filtering bleb, and the IOP can be elevated in some cases [456]. Combined cataract and glaucoma surgery may be advantageous for reducing the number of surgical procedures. However, several studies have reported that combined surgery is relatively ineffective in maintaining the filtering bleb and reducing the IOP compared with glaucoma filtering surgery alone [789].
If cataract surgery is performed first, it can improve the patient's vision without the risk of serious complications, such as hypotony, flat anterior chamber, choroidal detachment, and bleb leakage or blebitis, which can occur following filtering surgery [1011]. Previous studies have reported that phacoemulsification and intraocular lens implantation alone reduce IOP in both normal and glaucoma patients, and the IOP-lowering effects vary depending on the type of glaucoma and follow-up period [1213141516]. Patients with primary angle-closure glaucoma (PACG) show greater IOP reduction, with a concomitant decrease in the number of antiglaucoma medications in comparison with patients with primary open-angle glaucoma (POAG) after cataract surgery [17]. However, the IOP course can vary greatly after cataract surgery in glaucoma patients, so it is difficult to predict postoperative IOP in individual patients in clinical practice. It is important to therefore identify the factors associated with successful postoperative IOP control, which may be helpful in selecting the most appropriate surgical procedures for patients with coexisting visually significant cataract and glaucoma.
In a previous study, we studied POAG and PACG patients who underwent cataract surgery and divided them into success and failure groups according to the postoperative IOP and number of antiglaucoma medications used after surgery compared with baseline [18]. The failure group, who did not show significant IOP decrease after cataract surgery, had higher preoperative peak IOP values and were using more medications than the success group in both POAG and PACG patients, plus there was a larger area of peripheral anterior synechiae (PAS) in the PACG patients. The cutoff preoperative values for distinguishing success and failure were as follows: a preoperative peak IOP of 31 mmHg and use of three antiglaucoma medications before surgery in POAG patients and a preoperative peak IOP of 42 mmHg, the use of three medications, and a PAS area less than four clock hours in PACG patients.
In this study, we determined whether the aforementioned thresholds could be useful in predicting a favorable or poor postoperative IOP outcome and evaluated the number of antiglaucoma medications in use after cataract surgery. We compared the changes in IOP and number of antiglaucoma medications used between the two groups of glaucoma patients having cataract surgery alone, who were classified according to the criteria presented in our previous study [18].
Materials and Methods
Study design
We retrospectively analyzed the medical records of POAG and PACG patients with coexisting visually significant cataracts who underwent phacoemulsification and intraocular lens implantation at Chungnam National University Hospital between January 2007 and December 2013. This study was performed with approval from the institutional review board of Chungnam National University Hospital (2018-07-059) and adhered to the tenets of the Declaration of Helsinki.
A diagnosis of POAG was made for patients with an open anterior chamber angle, a pretreatment IOP >21 mmHg, and the presence of glaucomatous optic disc excavation or retinal nerve fiber layer defects accompanied by corresponding visual field defects. A diagnosis of PACG was based on the occludable angle (i.e., the presence of iridotrabecular contact for at least 180 degrees) on gonioscopic examination in accordance with the International Society of Geographical and Epidemiological Ophthalmology standard. PACG patients comprised those with an acute angle-closure attack expressed as a sudden IOP spike >50 mmHg, intermittent angle-closure glaucoma or chronic angle-closure glaucoma featuring a glaucomatous optic disc excavation or retinal nerve fiber layer defect, and corresponding visual field defects without any other pathological precedence factors. To be enrolled in this study, patients had to be followed for more than 36 months after surgery; the participants in this study were different from those included in our previous study [18]. If both eyes of a patient qualified for this study, one eye was selected at random. Patients with secondary glaucoma, a history of ocular trauma, posterior capsule rupture during phacoemulsification, or who underwent ocular surgery for ocular diseases other than glaucoma and cataract were excluded. If additional glaucoma surgery was performed for an uncontrolled IOP during the study period, the case was defined as an instance of failure, and only the data prior to the time of additional surgery were used in the analysis.
Surgical technique
All surgeries were performed by a single surgeon (CSK) under retrobulbar anesthesia. A temporal clear corneal incision was made using a 2.2-mm keratome blade, and the anterior chamber was filled with viscoelastic material (Healon; Abbott Medical Optics, Sunnyvale CA, USA). Then, a continuous curvilinear capsulorhexis, hydrodissection, and phacoemulsification using an Infiniti Vision System (Alcon, Fort Worth, TX, USA) were performed consecutively. A foldable intraocular lens (AcrylSof IQ SN60WF, Alcon) was inserted into the capsular bag. Following removal of viscoelastic material, the incision site was closed by one or two bites of 10/0 nylon suture. After surgery, 0.3% ofloxacin eye drops and 0.1% fluorometholone eye drops were instilled four times a day and gradually decreased over one month.
Data collection
The medical records of all patients were retrospectively reviewed and compared. All patients underwent preoperative ophthalmic examinations, including best-corrected visual acuity, slit-lamp biomicroscopy, gonioscopy, fundus examination, ocular biometry measurements, and visual field test using the Swedish Interactive Threshold Algorithm standard 24-2 perimetry (Humphrey Field Analyzer; Carl Zeiss Meditec, Dublin, CA, USA). Age, sex, type of glaucoma, presence of systemic disease, area of PAS, and mean deviation of Humphrey visual field tests were recorded. Gonioscopy was performed using a G-4 four mirror goniolens (Volk Optical, Mentor, OH, USA) before surgery to determine status of the angle and extent of PAS. Anterior chamber depth, lens thickness, and axial length were measured using ultrasound A-scan biometry (Ocuscan RxP, Alcon). Preoperative peak IOP was the highest recorded since the first examination by an ophthalmologist regardless of IOP-lowering treatment. In patients with acute angle-closure, the peak IOP was the highest observed during an acute attack. In addition, mean IOP (average IOP of the last two visits prior to surgery), number of antiglaucoma medications used, and cup-to-disc ratio before surgery were determined. After surgery, visual acuity, IOP, number of antiglaucoma medications, cup-to-disc ratio, and the type of complication (if any) were determined at 1, 3, 6, 12, 18, 24, and 36 months. IOP was measured using Goldmann applanation tonometry, and the average of two measurements was collected at each visit. Patients discontinued all antiglaucoma medications after surgery, and the same medications used prior to surgery were reintroduced one by one at the surgeon's discretion if the desired target IOP based on the patient's glaucoma status was not achieved. When counting the number of antiglaucoma medications, a fixed-combination drug was counted as two medications.
Patient classification
Primary glaucoma patients who underwent cataract surgery were divided into two groups based on preoperative factors, as described in our previous study [18]. Group 1 included POAG patients with a preoperative peak IOP <31 mmHg who were using fewer than three antiglaucoma medications used before surgery and PACG patients with a preoperative peak IOP <42 mmHg who were using fewer than three medications before surgery and who had an area of PAS less than four clock hours. Patients with levels exceeding these thresholds were classified into group 2.
Main outcome measures
Changes in IOP and number of medications and the success rate of postoperative IOP control were compared between the two groups for each type of glaucoma. Complete success was defined as a postoperative IOP of less than 21 mmHg and an IOP reduction of at least 20% from baseline without use of antiglaucoma medications, no significant complication that endangered visual function, and no need for additional glaucoma surgery. Qualified success was defined as a postoperative IOP less than baseline with use of the same or fewer medications compared to the number prior to surgery and the absence of any significant complications or need for additional surgery.
Statistical analysis
The PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for the analyses. Student's t-test was used to compare continuous variables, such as IOP values, numbers of medications, and PAS areas between the groups. The chi-square test was used to analyze nominal variables. Linear regression analysis was performed to determine the associations between preoperative factors and postoperative IOP changes. Kaplan-Meier survival analysis was used to compare the success of postoperative IOP control between group 1 and group 2. A p-value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
The study included 75 eyes of 75 patients with POAG and 95 eyes of 95 patients with PACG. Baseline characteristics of the study patients are shown in Table 1. The mean ± standard deviation age of the POAG patients was 66.9 ± 9.1 years (range, 47 to 81 years), and the mean follow-up period was 38.3 ± 3.2 months (range, 36 to 49 months). For the POAG patients, 33 eyes were classified as group 1 and 42 eyes were classified as group 2. The preoperative peak IOP in group 1 was significantly lower than that in group 2 (23.1 ± 2.4 vs. 30.1 ± 8.7 mmHg, p < 0.001). Separately, the mean age of the PACG patients was 66.4 ± 8.6 years (range, 48 to 87 years), and the mean follow-up period was 38.4 ± 3.7 months (range, 36 to 50 years). For the PACG patients, 43 eyes were classified as group 1 and 52 eyes were classified as group 2. The preoperative peak IOP of group 1 was significantly lower than that of group 2 (27.7 ± 8.3 vs. 44.0 ± 16.4 mmHg, p < 0.001). The area of the PAS was 0.7 ± 1.0 clock hours in group 1 and 1.0 ± 1.5 clock hours in group 2 (p = 0.205). No significant difference was found for age, sex, incidence of systemic disease, anterior chamber depth, lens thickness, axial length, mean deviation of the Humphrey visual field, and mean follow-up period between the two groups (all p > 0.05). In linear regression analysis, these factors were not associated with postoperative IOP changes in either group among either POAG or PACG patients.
Changes in IOP and medications in POAG patients
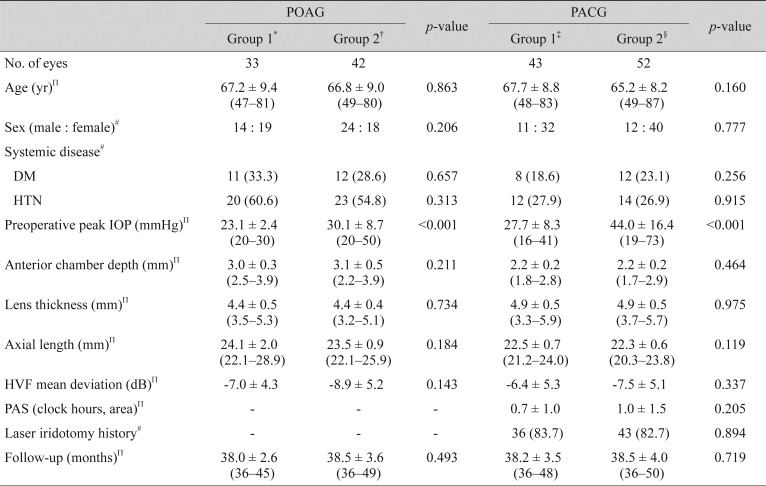
For the POAG patients, the mean IOP before surgery was 17.5 ± 1.8 mmHg in group 1 and 17.9 ± 1.9 mmHg in group 2. There was no significant difference in preoperative mean IOP between the two groups (p = 0.369, independent t-test). Following cataract surgery, the IOP decreased significantly throughout the study period in group 1, whereas group 2 showed a significant decrease at 12 months to 24 months after surgery (p < 0.05, paired t-test). Comparing the extent of change in IOP (baseline vs. after surgery) between the two groups, the reductions in IOP in groups 1 and 2 were 2.0 ± 2.1 and 0.6 ± 2.7 mmHg at 6 months, 2.0 ± 1.9 and 1.0 ± 2.2 mmHg at 12 months, 1.9 ± 2.0 and 0.9 ± 2.3 mmHg at 24 months, and 1.7 ± 2.1 and 0.6 ± 2.0 mmHg at 36 months after surgery, respectively; overall, group 1 showed a significantly greater reduction in IOP during the entire study period (all p < 0.05) (Table 2). Fig. 1 shows the mean percentage of IOP reduction from baseline in groups 1 and 2 in the POAG patients.

Comparison of changes in IOP and number of antiglaucoma medications after cataract surgery between groups 1 and 2 in primary open-angle glaucoma patients
The percentage of intraocular pressure (IOP) reduction from baseline (mean ± standard deviation) after cataract surgery at each follow-up visit in groups 1 and 2 in primary open-angle glaucoma patients (*p < 0.05, independent t-test).
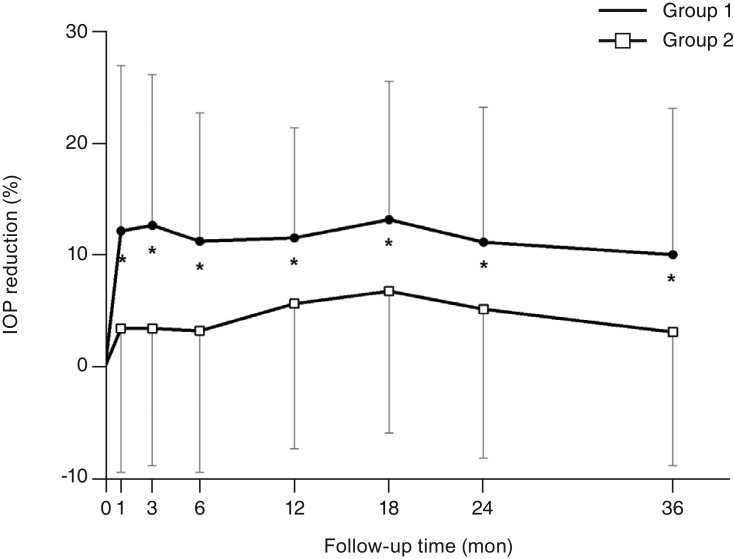
For the POAG patients, the number of antiglaucoma medications used before surgery was lower in group 1 (1.6 ± 0.6) than in group 2 (2.7 ± 0.9) (p < 0.001, independent t-test). The number of medications decreased significantly for 36 months after surgery in group 1 but only for 18 months in group 2 (p < 0.05, paired t-test). The reduction in number of medications used in groups 1 and 2 was not significantly different throughout the study period at 0.9 ± 0.8 and 0.9 ± 1.2 at 6 months, 0.8 ± 0.9 and 0.6 ± 1.1 at 12 months, 0.7 ± 1.0 and 0.4 ± 1.2 at 24 months, and 0.5 ± 1.0 and 0.2 ± 1.2 at 36 months after surgery, respectively (all p > 0.05) (Table 2).
Changes in IOP and medications in PACG patients
For the PACG patients, the mean IOP before surgery was not significantly different between groups 1 and 2 (17.5 ± 2.0 vs. 18.1 ± 2.6 mmHg, p = 0.222). The IOP after surgery was significantly lower than at baseline throughout the entire study period in both groups (p < 0.05). There was no significant difference in the extent of reduction in IOP between the two groups until 36 months after surgery; the IOP was reduced by 3.3 ± 2.1 and 2.5 ± 2.9 mmHg at 6 months, 2.9 ± 2.0 and 2.4 ± 2.8 mmHg at 12 months, 2.5 ± 1.9 and 2.2 ± 3.1 mmHg at 24 months, and 2.5 ± 2.0 and 2.2 ± 3.3 mmHg at 36 months in groups 1 and 2, respectively (all p > 0.05) (Table 3). Fig. 2 shows the mean percentage of IOP reduction from baseline in groups 1 and 2 in the PACG patients.
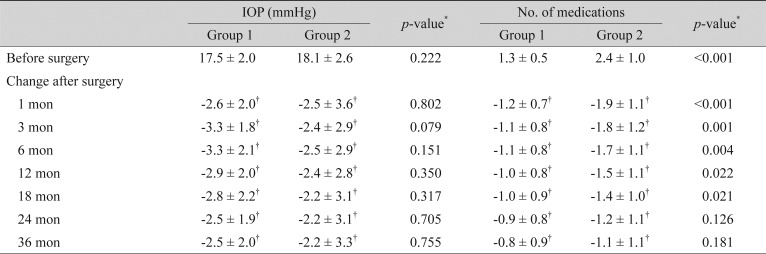
Comparison of changes in IOP and number of antiglaucoma medications after cataract surgery between groups 1 and 2 in primary angle-closure glaucoma patients
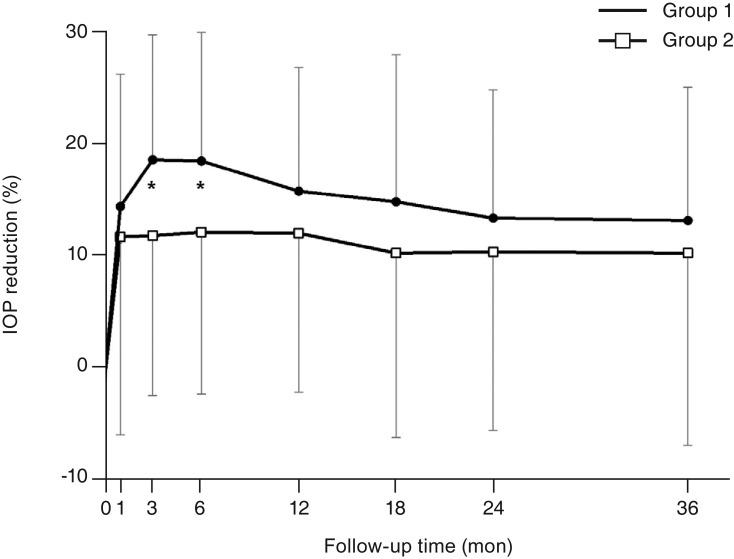
The percentage of intraocular pressure (IOP) reduction from baseline (mean ± standard deviation) after cataract surgery at each follow-up visit in groups 1 and 2 in primary angle-closure glaucoma patients (*p < 0.05, independent t-test).
For the PACG patients, the number of antiglaucoma medications used before surgery was lower for group 1 (1.3 ± 0.5) than for group 2 (2.4 ± 1.0) (p < 0.001). After surgery, the number of medications decreased significantly during the entire study period in both groups (p < 0.05). Group 2 showed a greater reduction in number of medications used for 18 months after surgery; however, there was no significant difference between the two groups after that until 36 months; the number of medications decreased by 1.1 ± 0.8 and 1.7 ± 1.1 at 6 months, 1.0 ± 0.8 and 1.5 ± 1.1 at 12 months, 0.9 ± 0.8 and 1.2 ± 1.1 at 24 months, and 0.8 ± 0.9 and 1.1 ± 1.1 at 36 months in groups 1 and 2, respectively (Table 3).
Success rates
In POAG patients, no eyes in group 1 underwent additional glaucoma surgery during the study period, while three eyes in group 2 required glaucoma filtering surgery (i.e., two eyes treated with trabeculectomy and one eye treated with Ahmed glaucoma valve implantation) to control the IOP. However, no PACG patient in either group required additional glaucoma surgery. In the analysis of complete success in the POAG patients, the success rates of groups 1 and 2 were 24.2% and 7.1% at 12 months, 18.2% and 4.8% at 24 months, and 15.2% and 2.4% at 36 months after surgery, respectively; overall, group 1 showed a significantly higher rate of complete success than did group 2 (p = 0.022, log-rank test). For the PACG patients, the complete success rates of groups 1 and 2 were 53.5% and 26.9% at 12 months, 37.2% and 19.2% at 24 months, and 20.9% and 17.3% at 36 months, respectively; there was no significant difference in the complete success rate between the two groups (p = 0.116, log-rank test) (Fig. 3A, 3B).

Kaplan-Meier survival curves of the complete success rate in (A) primary open-angle glaucoma and (B) primary angle-closure glaucoma. (A) The cumulative probability of success at 36 months was 15.2% in group 1 and 2.4% in group 2. The difference between the two groups was statistically significant (p = 0.022, log-rank test). (B) The cumulative probability of success at 36 months was 20.9% in group 1 and 17.3% in group 2. The difference between the two groups was not statistically significant (p = 0.116, log-rank test).
In the analysis of qualified success, the cumulative success rates in the POAG patients of groups 1 and 2 were 75.8% and 57.1% at 12 months, 69.7% and 42.9% at 24 months, and 66.7% and 35.7% at 36 months after surgery, respectively; group 1 showed a significantly higher rate of qualified success than did group 2 (p = 0.009). However, in the PACG patients, there was no significant difference in the qualified success rate between group 1 and group 2; the rates were 88.4% and 78.8% at 12 months, 83.7% and 73.1% at 24 months, and 79.1% and 69.2% at 36 months, respectively (p = 0.264) (Fig. 4A, 4B).
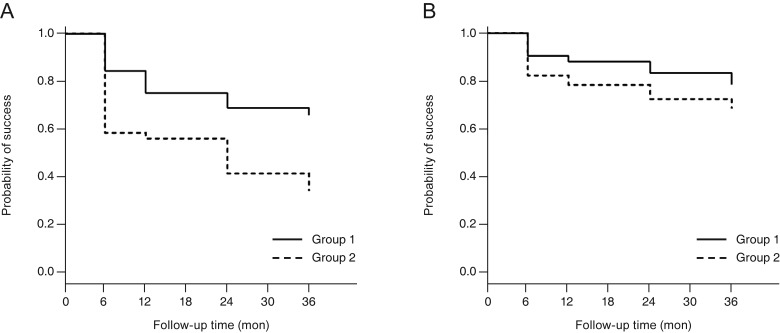
Kaplan-Meier survival curves of the qualified success rate in (A) primary open-angle glaucoma and (B) primary angle-closure glaucoma. (A) The cumulative probability of success at 36 months was 66.7% in group 1 and 35.7% in group 2. The difference between the two groups was statistically significant (p = 0.009, log-rank test). (B) The cumulative probability of success at 36 months was 79.1% in group 1 and 69.2% in group 2. The difference between the two groups was not statistically significant (p = 0.264, log-rank test).
Discussion
For patients with glaucoma and coexisting cataract, surgical treatments may be considered as a stepwise method, employing sequential treatment or combined operation for glaucoma filtering and cataract surgery. Glaucoma filtering surgery, such as trabeculectomy, is more effective in reducing IOP than cataract surgery but has a higher risk of complications such as a flat anterior chamber, hypotony, bleb leakage, and choroidal detachment [111920]; if cataract surgery is additionally performed after glaucoma surgery, proper functioning of the existing filtering bleb may be impaired or lost in some cases [45621]. Combined cataract and glaucoma surgery may result in a lower surgical success rate and unfavorable filtering bleb function (which may require more aggressive intervention to manage, such as antimetabolite injection) compared with glaucoma filtering surgery alone, possibly due to an excess inflammatory response with the combined procedure [2223242526]. In addition, for patients with angle-closure glaucoma, combined cataract and glaucoma surgery is associated with significantly greater risk for postoperative complications, and more pronounced IOP lowering is not always observed compared with cataract surgery alone [2728].
Cataract surgery in patients with glaucoma may temporarily increase the IOP in the early postoperative period, which could endanger the optic disc in advanced glaucoma patients [2930]. However, cataract surgery is usually safer than glaucoma surgery, improving visual function immediately and reducing the IOP to some extent; thus, it can be an effective surgical option in many patients.
Several studies have reported that cataract surgery alone decreases the IOP in glaucoma patients [121314151631], and that the IOP-lowering effect after cataract surgery differs according to the type of glaucoma [17]. Cataract surgery results in a modest reduction of IOP in eyes with OAG; however, the exact mechanism underlying the reduction of IOP remains unclear. Tong and Miller [32] reported that lens removal contracts the lens capsule, causing traction of the ciliary body through the zonules, which reduced aqueous humor production and lowered the IOP. As another potential mechanism, Handa et al. [33] found that changes in aqueous humor dynamics and the blood-aqueous barrier after cataract surgery might be related with changes in the IOP. Hayashi et al. [34] suggested that the width and depth of the anterior chamber angle increased not only in the ACG group, but also in the OAG group, leading to improved aqueous outflow. However, the ACG group showed greater changes in the anterior chamber angle and IOP compared to the OAG group. The main mechanism underlying ACG involves an anatomical factor, whereby the peripheral iris moves forward, resulting from increased lens thickness or from anterior lens position, which subsequently narrows the angle, appositional closure, or synechiae of the anterior chamber. Thus, removal of the lens by cataract surgery results in significant widening of the anterior chamber angle and increases aqueous outflow, suggesting that the IOP-lowering effect is greater in ACG patients than in OAG patients, who have a compromised trabecular meshwork rather than an anatomically narrow outflow pathway.
We verified that the PACG patients in this study showed a good efficacy regarding reductions in IOP and the number of medications used after cataract surgery compared to the POAG patients; this was consistent with the findings of previous studies [1718]. However, in a clinical setting, a wide variety of IOP responses are observed after cataract surgery, even for different patients with the same type of glaucoma. Therefore, it is important to identify the factors that can predict the IOP after cataract surgery and to determine whether to perform either combined cataract and glaucoma surgery or cataract surgery alone in patients with glaucoma and visually significant cataracts. In our previous study, we classified glaucoma patients who underwent cataract surgery into a success group and a failure group in terms of IOP control after surgery and analyzed the factors affecting the success of IOP control [18]. The success rate was most clearly differentiated when the subgroups were divided according to a preoperative peak IOP of 31 mmHg and use of three antiglaucoma medications before surgery in POAG patients and a preoperative peak IOP of 42 mmHg, use of three medications, and a PAS area of four clock hours in PACG patients.
In this study, we aimed to determine whether the cutoff point of our previous study had an effect in the manner proposed in actual clinical practice. Thus, we divided glaucoma patients with coexisting cataracts according to the aforementioned thresholds. Patients who were expected to show a favorable IOP course were classified into group 1 (preoperative peak IOP of less than 31 mmHg and <3 antiglaucoma medications used in POAG patients; preoperative peak IOP of less than 42 mmHg, <3 medications used, and a PAS area <4 clock hours in PACG patients). Patients who had an IOP, number of medications, or PAS area exceeding these thresholds and who would thus be expected to have a poorer IOP course were classified into group 2. We then compared the changes in IOP and number of medications used after cataract surgery in these patients, and the success of postoperative IOP control was analyzed in the context of a more detailed definition than that used in our previous study.
In POAG patients, the duration of significant IOP reduction after cataract surgery was longer and the extent of IOP reduction significantly greater in group 1 than in group 2 at 36 months after surgery. Although no significant difference was found in the change in number of antiglaucoma medications between the two groups, significant decrease in number of medications used relative to baseline persisted for up to 36 months in group 1 but only for up to 18 months in group 2. However, in PACG patients, there was no significant difference in the duration or extent of IOP reduction for up to 36 months after cataract surgery between the two groups. In addition, the number of medications used was significantly reduced from baseline during the entire follow-up period in both groups in PACG patients. These results indicated that cataract surgery was effective for IOP control in group 1, including both POAG and PACG patients. However, in group 2, the outcome of IOP control after cataract surgery was unfavorable in POAG patients, whereas it was relatively favorable in PACG patients.
In previous studies that analyzed factors affecting IOP after cataract surgery, the preoperative mean IOP was typically the most significant factor, and a higher mean IOP before cataract surgery was associated with a greater reduction in IOP after surgery [35363738]. However, Hayashi et al. [17] reported that patients with an uncontrolled IOP after cataract surgery had a significant higher preoperative IOP than did those in whom IOP control was successful, and Iancu and Corbu [39] noted that a higher preoperative IOP was associated with a failure of IOP control and a need for additional glaucoma surgery. These results imply that clinicians must consider a high IOP course after cataract surgery in spite of a greater IOP reduction in patients who have a high preoperative mean IOP. Several studies of PACG patients reported that anterior chamber depth before cataract surgery was associated with IOP changes after surgery, and a shallower anterior chamber was associated with a greater reduction in IOP after surgery [3840]. Other studies revealed a significant association of axial length or lens thickness with postoperative IOP change in nonglaucomatous eyes [4142], while Coh et al. [43] did not find a significant relationship between axial length or lens thickness and IOP reduction after cataract surgery in glaucomatous eyes. In the present study, no significant difference was found in the preoperative mean IOP, anterior chamber depth, axial length, or lens thickness between groups 1 and 2 for both POAG and PACG patients. Even if the aforementioned factors are similar between groups for each type of glaucoma, the IOP-lowering effect after cataract surgery may be different depending on other patient factors, such as peak IOP before surgery or the number of antiglaucoma medications required for IOP control at the time of surgery. Furthermore, the IOP course after cataract surgery in POAG patients was more affected by such factors than was that in PACG patients. PACG patients showed a significantly lower IOP course in both groups regardless of the peak IOP and the number of medications used before surgery, meaning that they were less affected by the mentioned factors.
In the analysis of success of IOP control, the complete success rate at 36 months was higher in group 1 than in group 2 in the POAG patients (15.2% vs. 2.4%). The relatively low complete success rate in this study may be due to the more stringent criteria used in comparison with in other studies of cataract surgery in glaucoma patients [1517]. Because the number of antiglaucoma medications used before surgery was significantly lower for group 1, the criteria for complete success (normal IOP and significant IOP reduction without using antiglaucoma medications) could be adversely applied to group 2, in which more medications were used before surgery. Therefore, we also analyzed the “qualified success” rate to evaluate more fairly the associations of these factors (i.e., the peak IOP and the number of medications before surgery) with the IOP course after cataract surgery. Qualified success was defined as a postoperative IOP lower than baseline with a similar or smaller number of antiglaucoma medications used after surgery versus baseline. In POAG patients, the qualified success rate at 36 months was also significantly higher in group 1 than in group 2 (66.7% vs. 35.7%). In addition, during the follow-up period, no eyes in group 1 required glaucoma surgery, whereas three eyes (7.1%) in group 2 underwent glaucoma filtering surgery. However, in the PACG patients, there were no statistically significant differences in the complete and qualified success rates between groups 1 and 2 (20.9% vs. 17.3% and 79.1% vs. 69.2%, respectively), and no eyes required additional glaucoma surgery during the study period in either group.
These results implied that IOP reduction after cataract surgery in group 2 POAG patients, who had higher preoperative peak IOP values and needed more antiglaucoma medications to control IOP before cataract surgery than did group 1, may have been associated with greater dysfunction in aqueous outflow, which may also have been restored less adequately (or unrestored) by cataract surgery than in group 1. However, the favorable IOP responses in group 2 PACG patients who had poor preoperative status could be explained by reversible dysfunction of aqueous outflow in PACG patients, at least in part, or at least less severe than that in POAG patients. We speculate that removal of the lens in cataract surgery results in widening of the anterior chamber angle and creation of more space in which the aqueous obtains access to the trabecular meshwork; this could provide better outflow, at least to some extent, even if the outflow facility is relatively compromised in PACG patients.
Our study was limited by the small number of patients included and the retrospective design. In addition, in POAG patients, although we tried to exclude patients showing uveitis by way of careful slit-lamp examination, we could not completely exclude secondary OAG conditions such as Posner-Schlossman syndrome from group 2, which had a relatively high peak IOP. The present study enrolled only glaucoma patients who underwent cataract surgery with an IOP moderately controlled by medication; we did not perform cataract surgery in patients with uncontrolled high IOP values for ethical reasons. Therefore, the results of this study may not be applicable to patients with uncontrolled IOP before surgery. An additional study to compare the IOP course between cataract surgery and combined glaucoma-cataract surgery in patients who are similar in terms of preoperative conditions may also help to elucidate the role of cataract surgery in IOP control. Also, a prospective study including a larger sample size and longer follow-up is needed.
In conclusion, this study suggested that the preoperative peak IOP and number of antiglaucoma medications used at the time of surgery, the predictive factors for IOP control after phacoemulsification delineated in our previous study, could affect the clinical outcomes of glaucoma patients treated with cataract surgery. In PACG patients, a favorable IOP course may be expected after cataract surgery alone, regardless of the level of preoperative peak IOP, number of medications used, and extent of PAS. However, if a POAG patient has a history of higher peak IOP and multiple antiglaucoma medications needed to control IOP before surgery, cataract surgery alone cannot guarantee a favorable IOP course, so additional measures such as combined cataract and glaucoma surgery may be considered for a favorable IOP course, especially when a low target IOP is required.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.