 |
 |
| Korean J Ophthalmol > Volume 32(6); 2018 > Article |
Abstract
Purpose
To investigate the efficacy, safety, and anatomical outcomes associated with intravitreal anti-vascular endothelial growth factor (VEGF) treatment of retinopathy of prematurity (ROP).
Methods
We performed a retrospective review of intravitreal anti-VEGF (bevacizumab or ranibizumab) treatment of 153 eyes (83 infants) diagnosed with ROP at two tertiary hospitals from June 2011 to January 2017. The primary outcome was the rate of recurrence requiring additional treatment; secondary outcomes included incidence of major complications and final refractive error.
Results
A total of 101 eyes were treated with bevacizumab, and 52 with ranibizumab. The bevacizumab and ranibizumab groups were characterized by mean birthweights of 941.8 ± 296.1 and 1,257.7 ± 514.5 g, gestational ages at birth of 26.9 ± 1.9 and 28.1 ± 3.2 weeks, and postmenstrual ages at treatment of 40.4 ± 2.4 and 39.2 ± 2.3 weeks, respectively. The two groups differed significantly in birthweights and gestational ages at birth, but not in postmenstrual ages at treatment. The mean follow-up duration was 30.9 ± 18.4 months for the bevacizumab group, and 13.9 ± 12.5 months for ranibizumab. More cases were classified as zone 1 ROP in the ranibizumab group (44.2% vs. 11.9%, p < 0.001). Major surgical interventions included scleral encircling and vitrectomy (one and two eyes, respectively, both in the bevacizumab group). Retinal detachment was noted in one eye treated with bevacizumab. There was no significant difference in the most recent spherical equivalence for the two groups (+0.10 ± 3.66 and +0.22 ± 3.00 diopters for bevacizumab and ranibizumab, respectively). Univariable analysis revealed that only ROP stage influenced the occurrence of major complications (odds ratio, 9.046; p = 0.012).
Retinopathy of prematurity (ROP) is a proliferative retinal vascular disease exclusive to premature infants, and a major cause of childhood blindness [1,2]. Blood vessel growth in immature retinas of premature infants is disrupted due to lung immaturity and respiratory distress syndrome [2,3]. Exposure to relative hyperoxia and subsequent hypoxia induces release of vascular endothelial growth factor (VEGF), causing abnormal blood vessel growth in the retina, leading to retinal detachment and severe visual disabilities [4,5]. ROP contributes to 3% to 10% of childhood blindness worldwide [6,7,8,9]. A survey of the Korean Neonatal Network database found that the total incidence rate of ROP was 34.1% among very-low-birth-weight infants, with 33.7% of these infants requiring treatment [10].
Following landmark studies such as the 2011 Bevacizumab Eliminates the Angiogenic Threat (BEAT)-ROP study [11], there has been widespread use of anti-VEGF injections as the primary treatment for zone 1 and posterior zone 2 ROP. The benefits of intravitreal anti-VEGF therapy include increased peripheral vascularization with resulting improvement in the visual field, less macular dragging, less pathological myopia, and the ease of the procedure without need for general anesthesia [12]. However, several studies have shown systemic suppression of VEGF and other growth factors after intravitreal anti-VEGF injection in neonatal infants [13,14], raising concerns about neurological or developmental delay [15]. There have also been isolated reports of retinal detachment [16,17,18] and endophthalmitis [19] following intravitreal injections.
Bevacizumab has been more widely reported than ranibizumab for treating ROP [11,20], but the use of ranibizumab has increased recently due to its theoretically safer systemic profile [21,22]. Bevacizumab is a humanized monoclonal murine antibody that binds all VEGF isoforms; ranibizumab is a humanized recombinant antibody fragment derived from bevacizumab, with approximately tenfold greater binding affinity [23]. Due to the smaller molecular weight of ranibizumab (48 vs. 149 kDa) and its shorter intravitreal half-life (26% that of bevacizumab) [24], ranibizumab is expected to clear faster from systemic circulation, potentially reducing systemic exposure and the risk of neurologic developmental defects [25].
In this study we compared the efficacy, anatomical outcomes, and rates of complications of anti-VEGF therapy for ROP using bevacizumab and ranibizumab.
This was a retrospective study conducted in two tertiary referral-based hospitals, Severance Hospital and Gangnam Severance Hospital, affiliated with Yonsei University College of Medicine. Patients diagnosed with ROP who received intravitreal anti-VEGF treatment from June 2011 to January 2017 were included, and their medical records were reviewed. All infants with ROP who required treatment were hospitalized in neonatal intensive care units. This study was conducted with the approval of our institutional review board (3-2018-0050) and adhered to the principles of the Declaration of Helsinki. The legal guardian of each patient signed a consent form before any examination or treatment.
Infants were screened for ROP if their gestational age at birth (GA) was <32 weeks and their birth weight (BW) was <1,500 g, or if they had an unstable clinical course as determined by the primary neonatologist. All examinations were performed by qualified ophthalmologists using the 2005 International Classification of Retinopathy of Prematurity [26]. Indications for treatment were infants who met criteria for type 1 ROP as defined in the ETROP (Early Treatment for Retinopathy of Prematurity) study [27], although earlier treatment was performed in some cases at the discretion of the primary ophthalmologist.
Intravitreal anti-VEGF—either bevacizumab (0.625 mg/0.025 mL; Avastin, Genentech, San Francisco, CA, USA) or ranibizumab (0.2 mg/0.02 mL; Lucentis, Novartis, Basel, Switzerland) [28,29]—was injected into each eye, with a gradual change in preference over the study period from bevacizumab to ranibizumab due to reports of safer systemic profiles for ranibizumab. The following techniques were used: topical anesthesia, sterile gloves, lid margin cleaned with povidone-iodine swabs, patient's face covered with a surgical towel with eye holes, insertion of a lid speculum, instillation of topical povidone-iodine, indentation of 0.5 to 1 mm posterior to the limbus with surgical calipers, injection of anti-VEGF with a sterile 30-gauge needle, removal of the needle with simultaneous compression using a sterile cotton tip, instillation of topical tobramycin, and removal of the speculum. New equipment was used for the fellow eye. Patients were reexamined the subsequent day and then weekly to monitor disease progression. Additional treatments such as additional injections, laser treatment, or surgeries such as vitrectomy and scleral encircling were performed as needed.
Patients were divided into bevacizumab and ranibizumab groups. The primary outcomes were ROP recurrences requiring retreatment, refractive errors, and major complications, defined as corneal opacity requiring transplantation, lens opacity requiring cataract surgery, retinal or vitreous hemorrhage requiring vitrectomy, retinal detachment, optic atrophy and glaucoma surgery, as well as major systemic complications such as death.
Statistical analysis was performed using IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA). Kolmogorov-Smirnov tests were used to analyze sample distributions. The independent t-test, the Mann-Whitney rank-sum test, the chi-square test, and the Fisher exact test were used. Logistic regression analyses were performed to assess the impact of patient and treatment factors on outcomes. A p-value <0.05 was considered statistically significant.
A total of 153 eyes from 83 infants were included in the study: 101 eyes treated with bevacizumab, and 52 with ranibizumab. The sex ratio, mean BW, GA, ROP classification, follow-up duration, and other baseline characteristics are shown in Table 1. The mean GA was 27.3 weeks overall, with a significant difference between the two groups (26.9 for bevacizumab, 28.1 for ranibizumab; p = 0.013). Accordingly, mean BW was higher in the ranibizumab group (1,257.7 vs. 941.8 g, p < 0.001). There was no difference in mean postmenstrual age at primary treatment, which was 40.0 weeks overall. There was no difference in the proportion of eyes classified as type 1 ROP (62.4% for bevacizumab, 55.8% for ranibizumab; p = 0.487) or in aggressive posterior ROP (5.0% for bevacizumab, 11.5% for ranibizumab; p = 0.186). There were more eyes with zone 1 ROP in the ranibizumab group (40.4% vs. 12.9%, p < 0.001). There was a significantly longer mean follow-up period for the bevacizumab group (30.9 vs. 13.9 months, p < 0.001).
ROP recurrences requiring additional treatment are summarized in Table 2. A total of 15 (9.8%) eyes had recurrences that required further intervention. Major interventions such as scleral encircling (one eye) or vitrectomy (two eyes) were required in a few cases in the bevacizumab group. Further treatment with laser photocoagulation was needed in one case following bevacizumab treatment. More eyes required additional anti-VEGF therapy following treatment with ranibizumab than bevacizumab (13.5% vs. 4.0%, p = 0.037).
Major complications and anatomical outcomes are shown in Table 3. Retinal detachment and temporal macular dragging each occurred in one eye in the bevacizumab group. Spherical equivalence at the most recent visit was +0.11 ± 3.58 diopters (D) overall, with no significant difference between the two groups (p = 0.922). There were more eyes with retinas fully vascularized to the ora serrata at follow-up in the bevacizumab group than in the ranibizumab group (100% vs. 85.0%, p < 0.001). The mean age at the most recent follow-up was 2.3 years old overall, and was higher in the bevacizumab group (2.8 vs 1.3 years, p < 0.001).
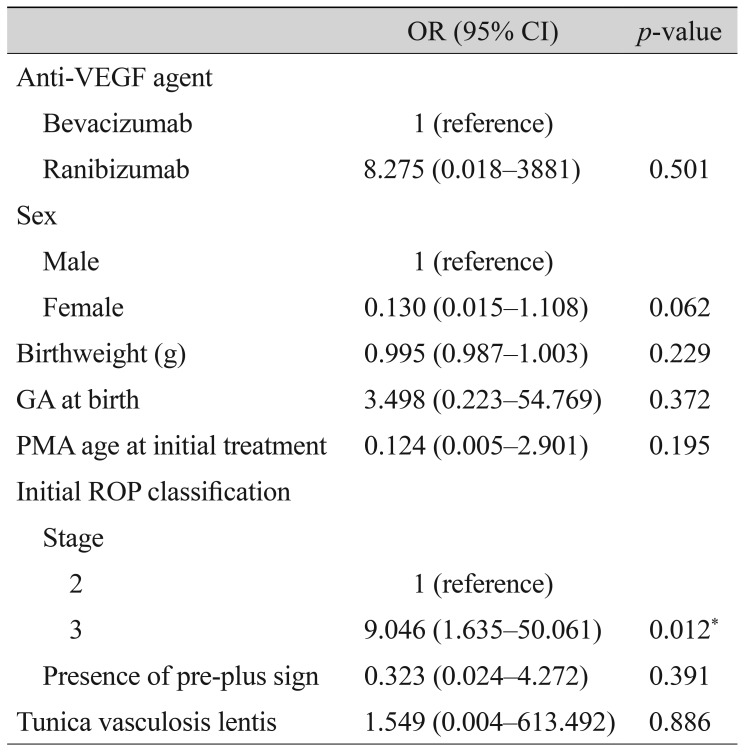
Univariable analysis of patient and treatment factors influencing the incidence of major complications revealed that only ROP stage was a significant factor (Table 4), with ROP stage 3 having a much higher odds ratio than ROP stage 2 (odds ratio, 9.046; p = 0.012). No patient or treatment factors were associated with retinal detachment (data not shown).
Anti-VEGF injections promote retinal vascularization without permanent destruction of the peripheral retina, and are less time-consuming or risky than conventional laser therapy. However, many ophthalmologists still question the use of anti-VEGF as the primary treatment for ROP, due to concerns regarding long-term complications. From January 2013 to June 2014 in Korea, of 231 very-low-birth-weight infant eyes treated for ROP, 63.6% received only an operation (laser, cryotherapy, or surgery), 16.9% received only anti-VEGF treatment, and 19.5% received both an operation and an anti-VEGF injection [10]. We investigated the efficacy, anatomical outcomes, and complications associated with intravitreal anti-VEGF ROP treatment using bevacizumab and ranibizumab.
In our study, 22% of cases of zone 1 ROP and 40% of “pre-plus” retinas were treated with anti-VEGF. These high anti-VEGF treatment rates may be due to a recent change in our centers to aggressively treating ROP with anti-VEGF at earlier time points. ROP is a biphasic disease, with a vaso-obliterative phase in which VEGF secretion is stimulated by relative hypoxia in the peripheral avascular retina, followed by a vasoproliferative phase [30,31]. A single burst of VEGF promotes vascular growth in ROP [32], which is different from other ocular neovascular diseases such as exudative neovascular age-related macular degeneration, in which there is continual VEGF release. Delayed anti-VEGF treatment, administered when VEGF levels are decreasing, may not be as effective and may promote fibrosis [33,34]. Accordingly, in our study the overall rate of ROP recurrence requiring additional treatment was 9.8%, lower than in previous studies (13% to 35.4%) [20,22,28].
Significantly more eyes required additional anti-VEGF treatment in the ranibizumab group. This may be because ranibizumab has a shorter intravitreal half-life than bevacizumab due to its smaller molecular weight [24]. The ranibizumab group also had a greater proportion of zone 1 ROP, which requires more time for full vascularization following initial anti-VEGF treatment. During the extended period of vascular growth, elevation in VEGF levels may reactivate ROP, requiring additional anti-VEGF treatment [35].
In terms of safety, anti-VEGF injections appear to cause few cases of retinal detachment or macular temporal dragging, as only one case of each occurred (both in the bevacizumab group). Furthermore, there was no significant difference in incidence of major complications following ranibizumab and bevacizumab treatments (odds ratio, 8.275; p = 0.501). Though both anti-VEGF agents enter systemic circulation following intravitreal injection [36,37], ranibizumab appears to suppress systemic VEGF for less time than bevacizumab, and to clear more rapidly from systemic circulation [36,38]. Therefore, ranibizumab is theoretically safer in its systemic profile, with less risk of neurologic deficits or developmental delays. In our analysis, more injections were required in the ranibizumab group, but there were cases in the bevacizumab group which required major interventions such as scleral encircling/buckling or vitrectomy (the number of cases was too small for statistical comparison). There were no major systemic complications in either group.
Full retinal vascularization to the ora serrata by the most recent follow-up was achieved in more cases in the bevacizumab group than in ranibizumab group (100% vs. 85.0%, p < 0.001). This may be due to the shorter follow-up period for the ranibizumab group (the mean age at the most recent follow-up was 2.3 years for bevacizumab, 1.3 years for ranibizumab; p < 0.001). There have been conflicting reports regarding the rate of retinal abnormalities and incomplete vascularization in long-term follow-up of ROP infants receiving anti-VEGF. For example, Castellanos et al. [39] followed six eyes of ROP patients receiving ranibizumab for three years and found fully vascularized retinas with no fluorescein leakage on angiography in all eyes. Isaac et al. [40] found peripheral vascularization to the ora serrata in four of five eyes that received bevacizumab, with one patient having too short a follow-up to determine vascularization potential properly. Huang et al. [22] noted incomplete vascularization without reactivation after ranibizumab treatment in 2.8% of 286 eyes. The BEAT-ROP study found the full vascularization rate to be 95.7% in 140 eyes. However, there have also been less favorable reports. Wu et al. [20] reported full vascularization in 88.0% of 162 bevacizumab-treated eyes, and Chen et al. [28] reported full vascularization in only 46.4% of 151 ranibizumab-treated eyes. In our study, we noted a full vascularization rate of 95.6% of 136 eyes receiving anti-VEGF treatment, with the remaining eyes having too short a follow-up period to properly determine the vascularization potential.
Refractive error at the most recent follow-up revealed association with mild hyperopia, with similar values for ranibizumab and bevacizumab. These results are consistent with previous results, which showed less association with myopia in anti-VEGF-treated eyes [12,41].
This study has several limitations. First, it was a retrospective study with a variable follow-up period and without appropriate controls; as such, significant differences in a few baseline characteristics between the treatment groups were noted. Second, the study population was limited to Asian patients in a tertiary hospital setting. Third, systemic complications may not have been properly observed or reported by the neonatologists. Last, we did not routinely perform fluorescein angiography to document fully vascularized retinas; however, the primary retina specialist performed thorough fundus examinations at each follow-up visit. The strengths of this study include the large number of patients with a mean follow-up period >12 months, and all patients being treated at only two hospitals using the same treatment protocols.
In conclusion, we found that intravitreal anti-VEGF injections achieve stable retinal vascularization with a low rate of complications and recurrences requiring additional treatment. Ranibizumab appears to achieve similar anatomical outcomes as bevacizumab, without additional risk for major complications such as retinal detachment or systemic side effects.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Blencowe H, Lawn JE, Vazquez T, et al. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013;74(Suppl 1):35-49.



2. Kushner BJ, Essner D, Cohen IJ, Flynn JT. Retrolental Fibroplasia. II. Pathologic correlation. Arch Ophthalmol 1977;95:29-38.


4. Casteels I, Cassiman C, Van Calster J, Allegaert K. Educational paper: retinopathy of prematurity. Eur J Pediatr 2012;171:887-893.


5. Malcolm W. Beyond the NICU: comprehensive care of the high-risk infant. New York: McGraw Hill Professional; 2014. p. 347-365.
6. Darlow BA. Retinopathy of prematurity: new developments bring concern and hope. J Paediatr Child Health 2015;51:765-770.


7. Goggin M, O'Keefe M. Childhood blindness in the Republic of Ireland: a national survey. Br J Ophthalmol 1991;75:425-429.



8. Hoogerwerf A, Schalij-Delfos NE, van Schooneveld MJ, Termote JU. Incidence of retinopathy of prematurity over the last decade in the Central Netherlands. Neonatology 2010;98:137-142.


9. Isaza G, Arora S, Bal M, Chaudhary V. Incidence of retinopathy of prematurity and risk factors among premature infants at a neonatal intensive care unit in Canada. J Pediatr Ophthalmol Strabismus 2013;50:27-32.


10. Hwang JH, Lee EH, Kim EA. Retinopathy of prematurity among very-low-birth-weight infants in Korea: incidence, treatment, and risk factors. J Korean Med Sci 2015;30(Suppl 1):S88-S94.



11. Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603-615.



12. Geloneck MM, Chuang AZ, Clark WL, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol 2014;132:1327-1333.


13. Kong L, Bhatt AR, Demny AB, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci 2015;56:956-961.


14. Wu WC, Lien R, Liao PJ, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol 2015;133:391-397.


15. Morin J, Luu TM, Superstein R, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 2016;3 17

16. Jang SY, Choi KS, Lee SJ. Delayed-onset retinal detachment after an intravitreal injection of ranibizumab for zone 1 plus retinopathy of prematurity. J AAPOS 2010;14:457-459.


17. Yonekawa Y, Wu WC, Nitulescu CE, et al. Progressive retinal detachment in infants with retinopathy of prematurity treated with intravitreal bevacizumab or ranibizumab. Retina 2018;38:1079-1083.


18. Lee BJ, Kim JH, Heo H, Yu YS. Delayed onset atypical vitreoretinal traction band formation after an intravitreal injection of bevacizumab in stage 3 retinopathy of prematurity. Eye (Lond) 2012;26:903-909.



19. Wang J, Xiang D. Early clinical characteristics of bacterial endophthalmitis in retinopathy of prematurity after intravitreal bevacizumab injection: a case report. Exp Ther Med 2017;13:3563-3566.



20. Wu WC, Kuo HK, Yeh PT, et al. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in Taiwan. Am J Ophthalmol 2013;155:150-158.


21. Chen SN, Lian I, Hwang YC, et al. Intravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between ranibizumab and bevacizumab. Retina 2015;35:667-674.


22. Huang Q, Zhang Q, Fei P, et al. Ranibizumab injection as primary treatment in patients with retinopathy of prematurity: anatomic outcomes and influencing Factors. Ophthalmology 2017;124:1156-1164.


23. Csaky K, Do DV. Safety implications of vascular endothelial growth factor blockade for subjects receiving intravitreal anti-vascular endothelial growth factor therapies. Am J Ophthalmol 2009;148:647-656.


24. Semeraro F, Morescalchi F, Duse S, et al. Aflibercept in wet AMD: specific role and optimal use. Drug Des Devel Ther 2013;7:711-722.



25. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859-870.


26. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991-999.


27. Good WV. Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233-248.


28. Chen Y, Fen J, Meng X. Effects of ranibizumab in zone I and zone II retinopathy of prematurity patients. Chinese J Ophthalmol 2015;31:6-9.
29. Darlow BA, Ells AL, Gilbert CE, et al. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed 2013;98:F170-F174.


31. Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med 2012;367:2515-2526.



32. Micieli JA, Surkont M, Smith AF. A systematic analysis of the off-label use of bevacizumab for severe retinopathy of prematurity. Am J Ophthalmol 2009;148:536-543.


33. Klaassen I, van Geest RJ, Kuiper EJ, et al. The role of CTGF in diabetic retinopathy. Exp Eye Res 2015;133:37-48.


34. Hartnett ME. Vascular endothelial growth factor antagonist therapy for retinopathy of prematurity. Clin Perinatol 2014;41:925-943.



35. Geisen P, Peterson LJ, Martiniuk D, et al. Neutralizing antibody to VEGF reduces intravitreous neovascularization and may not interfere with ongoing intraretinal vascularization in a rat model of retinopathy of prematurity. Mol Vis 2008;14:345-357.


36. Hong YR, Kim YH, Kim SY, et al. Plasma concentrations of vascular endothelial growth factor in retinopathy of prematurity after intravitreal bevacizumab injection. Retina 2015;35:1772-1777.


37. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014;98:1636-1641.



38. Hoerster R, Muether P, Dahlke C, et al. Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol 2013;91:e74-e75.


39. Castellanos MA, Schwartz S, Garcia-Aguirre G, Quiroz-Mercado H. Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br J Ophthalmol 2013;97:816-819.


Table 2
Retinopathy of prematurity recurrence requiring additional treatment after intravitreal anti-VEGF
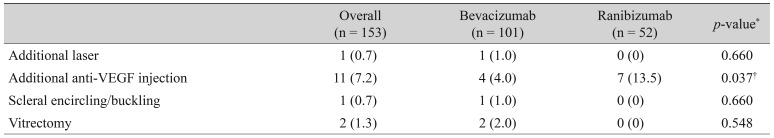
Table 3
Major complications and anatomical outcomes after anti-vascular endothelial growth factor injection for retinopathy of prematurity

- TOOLS
-
METRICS

-
- 21 Crossref
- 0 Scopus
- 2,732 View
- 38 Download
- Related articles




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



