Efficacy and Safety of Intracameral Bevacizumab for Treatment of Neovascular Glaucoma
Article information
Abstract
Purpose
To evaluate the long-term efficacy and safety of intracameral bevacizumab in patients with neovascular glaucoma.
Methods
This retrospective study included 26 eyes of 26 neovascular glaucoma patients who received intracameral bevacizumab injection between January 2013 and May 2015, and were followed-up for at least 1 year. All patients were treated with topical and/or systemic intraocular pressure (IOP)-lowering medications, intracameral bevacizumab, and panretinal photocoagulation (PRP). The main outcome measures were changes in visual acuity, IOP, and neovascularization of the iris (NVI) and the anterior chamber angle (NVA). To assess the safety of intracameral bevacizumab, corneal endothelial changes were also determined using specular microscopy. Patients whose IOP was uncontrolled received IOP-lowering surgery. Clinical factors associated with IOP-lowering surgery were also investigated.
Results
In all patients, intracameral bevacizumab resulted in a rapid and marked reduction of IOP, NVI, and NVA within 1 week. At 12 months after initial injection, 19 of 26 eyes (73%) underwent IOP-lowering surgery. The average interval between initial injection and surgical treatment was 33.6 ± 26.9 days. Baseline IOP (p = 0.018), NVA grade (p = 0.029), and incomplete PRP (p = 0.005) were identified as predictive factors for IOP-lowering surgery. During the follow-up period, there were no statistically significant corneal endothelial changes after intracameral bevacizumab injection.
Conclusions
During 1 year of follow-up after intracameral bevacizumab, the procedure was found to be safe for the corneal endothelium. However, the IOP-lowering effect was transient, and 73% of patients eventually required IOP-lowering surgery. Predictive factors for IOP-lowering surgery were high baseline IOP and NVA grade, and incomplete PRP.
Neovascular glaucoma (NVG) is a secondary glaucoma, resulting from ocular ischemia caused by various diseases such as proliferative diabetic retinopathy (PDR), retinal vein occlusion, ocular ischemic syndrome, and chronic uveitis [1]. Ischemia-induced abnormal fibrovascular tissue grows on the iris and anterior chamber angle and progresses to produce obstruction of the trabecular meshwork, Peripheral anterior synechiae (PAS), and angle closure. These histopathological changes eventually result in elevated intraocular pressure (IOP) and severe vision loss.
There are two goals for management of NVG: 1) treat the underlying condition responsible for the neovascular stimulus, and 2) control the elevated IOP, which results in progressive optic neuropathy if untreated. Panretinal photocoagulation (PRP) is the standard treatment for ablation of the ischemic retina and induces involution of the vasoproliferative pathology that includes abnormal blood vessel growth on the retina, iris, and anterior chamber angle. However, the manifestation of the anti-neovascular effect driven by PRP requires several weeks, and PRP usually does not induce prompt regression of iris and angle neovascularization. Therefore, ocular damage may persist due to elevated IOP in the eyes of NVG patients [23]. Moreover, adequate laser treatment is often difficult due to media opacity caused by corneal edema, hyphema, cataracts, and/or vitreous hemorrhage. Vascular endothelial growth factor (VEGF), induced by ocular ischemia, and plays a central role in ocular neovascularization and NVG [45]. Aqueous levels of VEGF are highly elevated and significantly correlated with the extent of neovascularization and IOP in NVG patients [67]. Based on clinical and experimental findings, anti-VEGF therapy was proposed as another therapeutic option in NVG [8].
Bevacizumab (Avastin; Genentech, South San Francisco, CA, USA), a humanized monoclonal antibody against all VEGF isoforms, is widely used to treat VEGF-mediated ocular conditions, such as choroidal neovascularization, age-related macular degeneration, and diabetic macular edema. Bevacizumab is also used as an adjunctive agent in glaucoma surgery. Previous studies have reported that intraocular bevacizumab injection can cause a rapid and marked regression of neovascularization of the iris (NVI) and the anterior chamber angle (NVA), and can reduce IOP in early-stage NVG. Wolf et al. [9] reported complete resolution of NVI and IOP reduction in all patients 1 week after intracameral bevacizumab. Sasamoto et al. [6] found a significant reduction of NVI, NVA, and IOP 1 week after intravitreal bevacizumab.
However, the effect of intraocular bevacizumab for NVG may be transient. The half-life of intravitreal bevacizumab was reported to be only 9.8 days in humans and 4.32 days in animals [910]. Wolf et al. [9] demonstrated that the effect of intracameral bevacizumab lasted approximately 23 days, and 6 months after injection, 21 of 24 (87.5%) eyes required additional treatment, such as repeated injection or PRP. Yazdani et al. [11] also reported that 12 of 14 eyes (85.7%) required additional laser or surgical procedures 6 months after intravitreal bevacizumab.
Although the rapid and obvious therapeutic effects of intraocular bevacizumab have been reported in many studies, little is known about the long-term efficacy of intraocular, especially intracameral, injection. In addition, previous studies have reported that most patients eventually required laser or surgical procedures for IOP control, but there have been very few studies on the predictive factors for surgical treatment in spite of intraocular injection, especially intracameral injection [12]. Therefore, the purpose of the present study was to observe the long-term efficacy and safety of intracameral bevacizumab and to investigate the clinical factors associated with IOP-lowering surgery, despite the use of intracameral bevacizumab in NVG patients.
Materials and Methods
This study was approved by the institutional review board of Chonnam National University Hospital (CNUH-2017-017) and is in accordance with the principles of the Declaration of Helsinki. The authors retrospectively reviewed the medical records of 26 eyes of 26 patients who underwent intracameral bevacizumab treatment for NVG between January 2013 and May 2015.
NVG was defined as IOP >21 mmHg with the presence of NVA or NVI. Patients previously treated with intraocular anti-VEGF injection or anti-glaucoma surgery, and those with severe media opacity preventing examination of the anterior segment were excluded.
All subjects received anti-glaucoma topical and/or systemic medications, intracameral bevacizumab, and PRP. PRP was performed with a slit lamp using a 532-nm green laser (OcuLight GL; Iridex, Mountain View, CA, USA). A level II to III reaction for retinal photocoagulation was appropriate for laser output power intensity, the spot size was 300 to 500 microns, the exposure time was 0.1 to 0.2 seconds, and the photocoagulation scope was 0.5 to 1.0 papilla diameter (PD) on the nasal side in the optic disc and 2 PD on the temporal side in the macula and the left retina outside of both the up and down vascular arcades. Depending on the view of the retina and patient tolerance, PRP was done over 1 to 3 sessions. Supplemental PRP was added to areas of nonperfusion revealed by fundus fluorescein angiography (FFA) at 1 to 2 months after the first PRP. We defined complete PRP as all of the NVI and NVA were regressed after PRP or all of the nonperfusion area was laser-ablated so there was no need for additional photocoagulation.
During follow-up, intracameral bevacizumab was repeated if IOP had increased to >21 mmHg despite medical and laser treatment, or if there was a prominent recurrence of NVI and/or NVA. Despite all efforts to control IOP, IOP-lowering surgery, such as trabeculectomy with mitomycin C, Ahmed valve implantation, or trans scleral cyclophotocoagulation, was performed by a single glaucoma specialist (SWP) when IOP was above the target with progression of glaucomatous optic neuropathy. The target IOP was estimated to prevent further nerve damage and was set for each patient based on their initial IOP and degree of existing damage.
Intracameral bevacizumab injection was performed at an outpatient clinic. Informed consent was obtained from each patient prior to the procedure. After aseptic preparation (5% povidone-iodine solution) and application of topical anesthetic eye drops (proparacaine hydrochloride 0.5%; Alcaine, Alcon, Fort Worth, TX, USA), bevacizumab solution (25 mg/mL, 0.05 mL) was injected at the limbus in the temporal quadrant, using a 30-gauge needle after paracentesis. Before injection, paracentesis (0.1 to 0.2 mL) was performed to prevent IOP elevation due to the injected solution [12].
All subjects underwent comprehensive ophthalmologic examinations, including detailed medical histories, measurements of best-corrected visual acuity (BCVA) by logarithm of the minimum angle of resolution (logMAR) scale, anterior segment and fundus examinations with slit-lamp biomicroscopy, IOP measurements by Goldmann applanation tonometry, anterior chamber angle examinations with Posner four-mirror goniolens, specular microscopy, and dilated fundus exam with FFA. The medical records of the patients were reviewed for age, sex, BCVA, preexisting ischemic ocular disorders, the number of topical anti-glaucoma medications, and prior treatments. NVI and NVA grade by Weiss and Gold classification were also assessed, which distinguish four stages of neovascularization, according to the area of new vessels in the iris, anterior chamber angle, and the location of PAS [13]. Grading of NVI and NVA was performed by a single glaucoma specialist (SWP). NVI grading is as follows: grade 1, fine surface neovascularization of the pupillary zone of the iris, involving ≤2 quadrants; grade 2, surface neovascularization of the pupillary zone of the iris, involving ≥2 quadrants; grade 3, in addition to the pupillary zone, neovascularization of the ciliary zone of the iris and/or ectropion uveae involving 1 to 3 quadrants; grade 4, neovascularization of the ciliary zone of the iris and/or ectropion uveae involving ≥3 quadrants. NVA grading is as follows: grade 1, fine neovascular twigs cross scleral spur and ramify on trabecular meshwork, involving ≤2 quadrants; grade 2, neovascular twigs cross scleral spur and ramify on trabecular meshwork, involving ≥2 quadrants; grade 3, in addition to the trabecular meshwork, PAS involving 1 to 3 quadrants; grade 4, PAS involving ≥3 quadrants.
All patients were divided into two groups, patients who received IOP-lowering surgery and patients whose IOP was controlled without surgery. The changes in BCVA, IOP, NVI, and NVA were acquired and compared between the two groups.
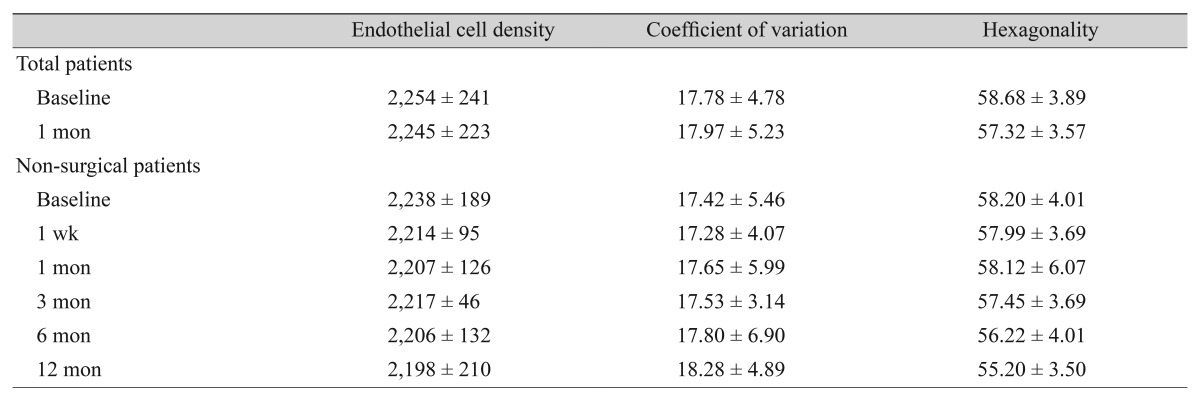
The frequency of IOP-lowering surgeries during follow-up, the number of injections, the number of patients receiving multiple injections, the interval between initial injection and surgery, the type of surgery, and predictive factors for surgery were also assessed. In addition, endothelial cell counts, coefficient of variation, and hexagonality were evaluated via specular microscopy to assess corneal endothelial toxicity due to the injected bevacizumab.
Statistical analysis was performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Pre-injection baseline BCVA, IOP, NVI, and NVA were compared with post-injection follow-up examination values using paired t-tests and the paired McNemar test. The Mann-Whitney U-test and chi-square test (or Fisher's exact test) were performed to compare the baseline characteristics and treatment outcomes between the two groups. Logistic regression analysis was performed to examine the predictive factors for IOP-lowering surgery. Paired t-tests were also used to evaluate corneal endothelial changes after intracameral bevacizumab injection. A p-value of <0.05 was considered statistically significant.
Results
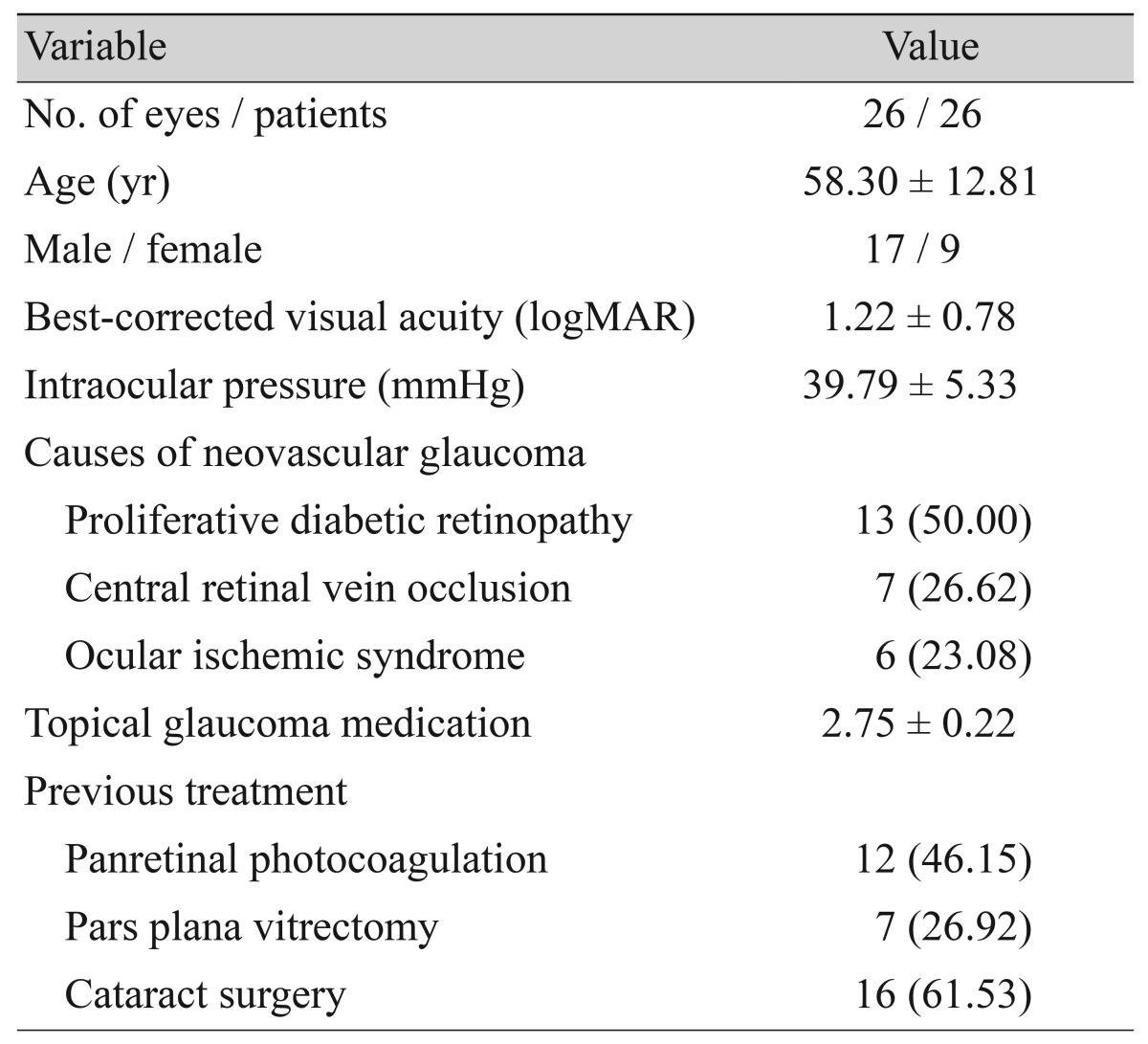
The baseline characteristics of included patients are summarized in Table 1. From a total of 34 eyes of 34 patients who received intracameral bevacizumab, 26 eyes of 26 patients were included in this study. Of the eight eyes excluded from analyses, four eyes had not been followed-up for 12 months, and four eyes could not have the anterior chamber of the eye examined due to severe corneal edema or hyphema. Of the 26 included patients, 17 were men and 9 were women. The mean age was 58.3 ± 12.8 years (range, 42 to 78 years). At the initial visit, BCVA was 1.2 ± 0.8 logMAR, and IOP was 39.8 ± 5.3 mmHg, respectively. An average of 2.8 ± 0.2 topical anti-glaucoma medications were administered. The underlying ophthalmic conditions causing NVG were PDR (13 eyes, 50%), central retinal vein occlusion (7 eyes, 26%), and ocular ischemic syndrome (6 eyes, 23%). Before receiving intracameral bevacizumab injection, 12 (46%), 7 (26%), and 16 (61%) eyes had undergone PRP, pars plana vitrectomy (PPV), and cataract surgery, respectively.
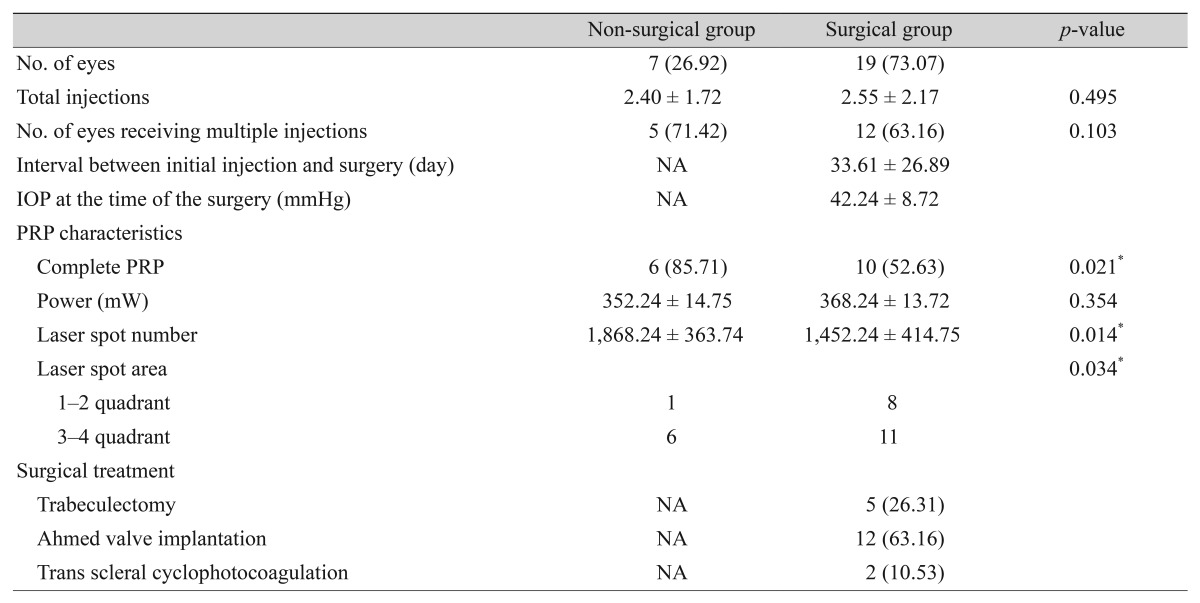
The details of therapeutic intervention in the non-surgical and surgical group are summarized in Table 2. During the entire follow-up period, 19 patients received surgical treatment. The surgical procedures were as follows: five eyes (26%) underwent trabeculectomy with mitomycin C, 12 eyes (63%) underwent Ahmed valve implantation with mitomycin C, and two eyes (10%) underwent trans scleral cyclophotocoagulation. The total number of injections was 2.40 ± 1.72 in the non-surgical group and 2.55 ± 2.17 in the surgical group (p = 0.495). Five eyes (71%) in the non-surgical group and 12 eyes (63%) in the surgical group required a repeated injection (p = 0.103). During the follow-up period, complete PRP was performed in 6 eyes (85%) in the non-surgical group and 10 eyes (52%) in the surgical group (p = 0.021). The non-surgical group received more spots and quadrant areas than the surgical group (p = 0.014 and p = 0.034, respectively). There was no difference in laser power.
The change in IOP after intracameral bevacizumab is shown in Fig. 1. At 1 week, IOP was stabilized to 16.5 ± 3.4 mmHg in 22 of 26 eyes. However, 4 of 26 eyes required IOP-lowering surgery and IOP was reduced to 12.0 ± 2.8 mmHg at 1 week after injection. Despite intracameral injections and other medical treatments, the number of eyes requiring additional surgical treatment were increased and 14 (53%), 16 (62%), and 19 eyes (73%) had received IOP-lowering surgeries at 1, 6, and 12 months after injection, respectively. Nevertheless, both groups showed successful IOP normalization (<20 mmHg) during a 1-year follow-up period.
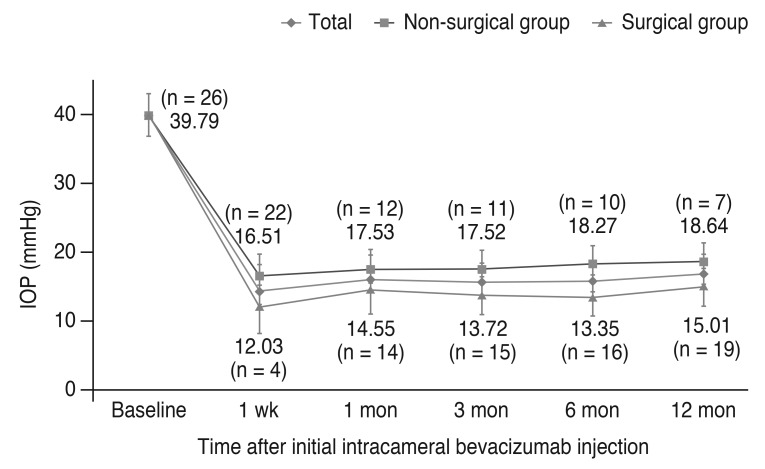
The changes in intraocular pressure (IOP) after intracameral bevacizumab injection. Of 26 eyes, IOP in 22 eyes could be controlled with injection, but 4 eyes received anti-glaucoma surgery 1 week after injection. At 1 month, 14 eyes received the surgery, and the number increased to 19 eyes at 12 months after injection. IOP in the eyes of the non-surgical group was maintained <20 mmHg after treatment. The surgical group showed poor response to injection, but, after surgery IOP also stabilized.
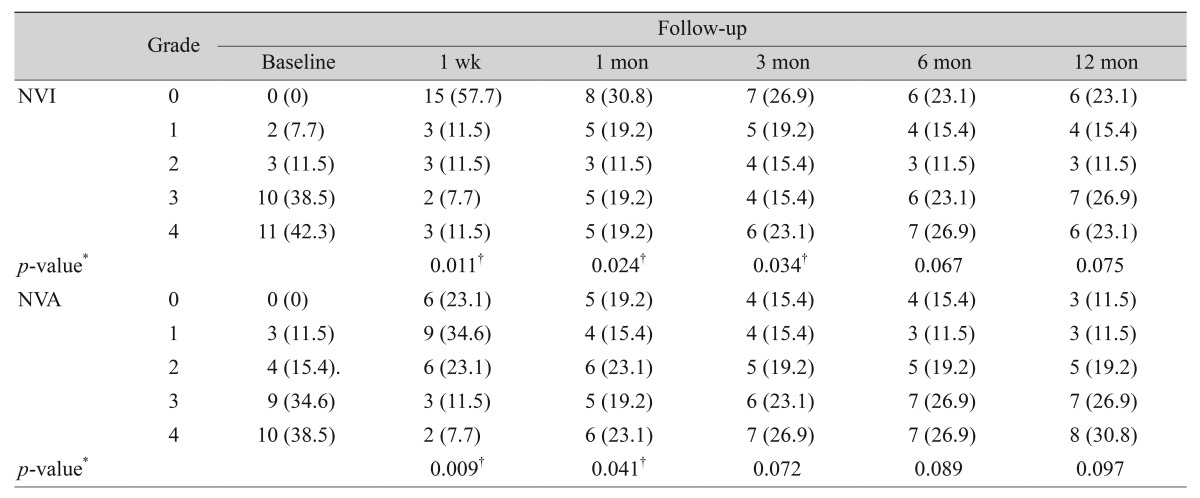
Serial change in NVI and NVA grade in total patients during the follow-up period is shown in Table 3. More than 70 % of eyes were distributed as advanced grade (i.e., 3 or 4) in NVI and NVA at baseline. NVI and NVA rapidly regressed after injection. NVI disappeared in 15 eyes (58%) and NVA disappeared in 6 eyes (23%) and eyes with advanced grade in NVI and NVA were reduced to 5 eyes, respectively (18%) (p = 0.01 in NVI and NVA) 1 week after injection. This trend continued in both NVI and NVA 1 month after injection (p = 0.02 and p = 0.04, respectively). However, at 3 months post-injection, the effect of intracameral injection was maintained in NVI (p = 0.03) but not in NVA (p = 0.07). Intra-rater reliability for NVI and NVA grading was evaluated by calculation of Cohen's kappa coefficient. Coefficient values were 0.86 (95% confidence interval [CI], 0.77 to 0.91) for NVI grading and 0.87 (95% CI, 0.74 to 0.90) for NVA grading. The kappa values for both grades were up to 0.9, which was sufficient to ensure reasonable reliability [14].
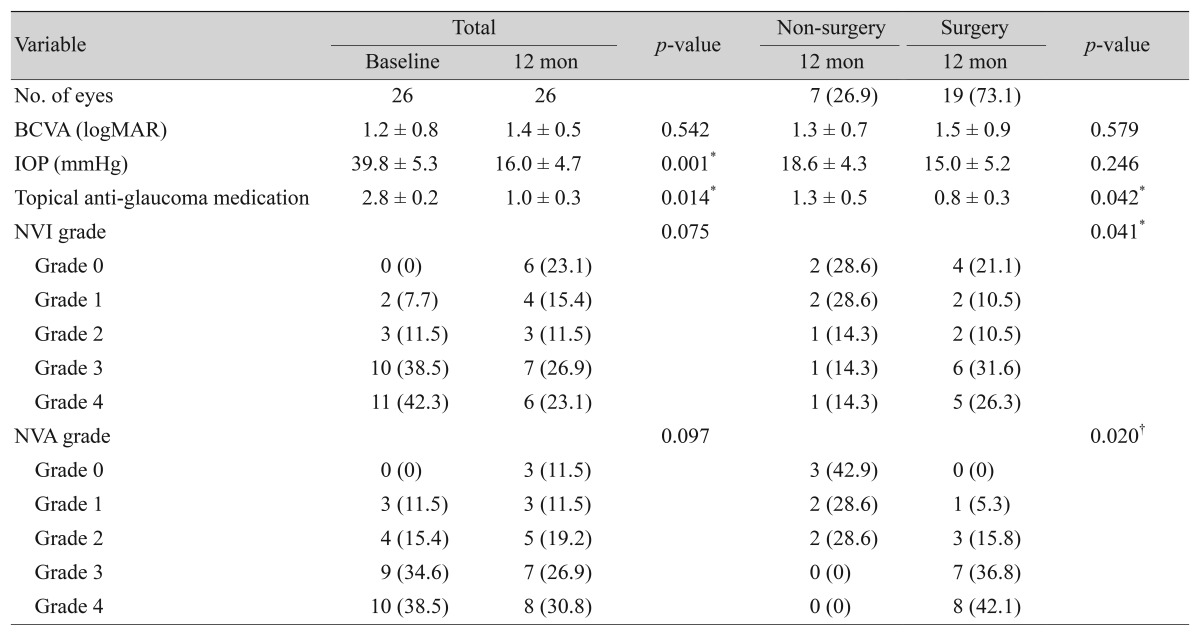
Treatment results at 12-month follow-up are summarized in Table 4. BCVA remained relatively stable during the course of treatment (1.2 ± 0.8 vs. 1.4 ± 0.5 logMAR at baseline and 12-month follow-up, respectively; p = 0.542). There was a reduction in IOP and the number of topical anti-glaucoma medications after treatment (39.8 ± 5.3 vs. 1.4 ± 0.5 mmHg, 2.8 ± 0.2 vs. 1.0 ± 0.3 at baseline a nd 12-month follow-up, respectively; all p < 0.05). Comparing the non-surgical and the surgical groups, there were no differences in BCVA or IOP at 12-month follow-up. IOP was stabilized in both groups, but the non-surgical group was treated with a greater average number of medications than the surgical group (1.3 ± 0.5 vs. 0.8 ± 0.3, p < 0.05). In addition, NVI and NVA were more advanced in the surgical group than in the non-surgical group (p = 0.041 and p = 0.020, respectively).
The predictive factors for IOP-lowering surgical treatments were analyzed, and the results are shown in Table 5. Multivariate logistic regression analysis showed that baseline IOP (p = 0.018; odds ratio [OR], 4.45; 95% CI, 2.79 to 12.51) and complete PRP (p = 0.005; OR, 0.28; 95% CI, 0.09 to 0.73) were strong predictors for IOP-lowering surgery. Notably, the odds of IOP-lowering surgery increased as baseline grade of NVA increased and were 7.01 times higher in patients with grade 4 than in patients with grade 1 (95% CI, 2.65 to 30.67) (Table 5).
Regarding the cytotoxicity of intracameral bevacizumab on the corneal endothelium, cell density, coefficient of variation, and hexagonality of corneal endothelial cells 1 month after injection did not significantly change compared to baseline in all patients. For exclusion of the effect of surgery on corneal endothelium, the same parameters only in the non-surgical group were analyzed, and there was no obvious corneal endothelial damage at 12-month follow-up, despite receiving multiple intracameral bevacizumab injections. Comparing baseline values to those at 12 months in the non-surgery patients, there were no statistical differences in endothelial cell density (2,238 ± 189 vs. 2,198 ± 210, p = 0.120), coefficient of variation (17.4 ± 5.5 vs. 18.3 ± 4.9, p = 0.267), and hexagonality (58.2 ± 4.0 vs. 55.0 ± 3.5, p = 0.421) (Table 6).
Discussion
The definitive treatment for NVG is the elimination of neovascular stimuli by ablation of the ischemic retina, thereby reducing vasoproliferative factors (e.g., VEGF) and abnormal new vessel formation. Although the effect of retinal ablation is definitive and long lasting, it often takes several weeks to manifest [3]. During this period, progressive angle closure and optic nerve damage may persist due to sustained VEGF and IOP elevation, and may result in painful vision loss. Because of its rapid and potent effect of directly targeting VEGF, intraocular bevacizumab was established as a standard protocol for NVG treatment [15]. However, most of the patients who received intraocular bevacizumab required additional injections, PRP, or in the long-term, surgical treatment for IOP control [12161718]. Generally, a drug with intracameral injection shows shorter half-life and duration of effect than those administered via intravitreal injection. However, intracameral injection can be performed in the presence of media opacities, has fewer vitreoretinal complications, and shows better IOP-lowering effects than intravitreal injection in some reports [816]. Therefore, this study investigated the long-term efficacy and safety of intracameral bevacizumab injections and the predictive factors for eventual IOP-lowering surgery.
Previous studies have reported the rapid and marked reduction of abnormal new vessels and IOP after intraocular bevacizumab in NVG patients [69]. Our study also showed a similar beneficial effect in NVG patients. IOP, NVI, and NVA rapidly decreased within 1 week, and the effect continued to 1 month in NVA, and 3 months in NVI. However, despite multiple intracameral bevacizumab injections, the number of patients who required IOP-lowering surgery increased during the follow-up period. Consequently, 19 of 26 eyes (73%) had received IOP-lowering surgery by 12 months. Of 19 eyes that received IOP-lowering surgery, the majority of eyes (15 eyes, 79%) received surgery within the first 3 months after initial injection. It is very unlikely that the effect of intracameral bevacizumab (ICB) in controlling IOP would be maintained over 3 months. In addition, there was a reduction in the number of topical anti-glaucoma medications in both groups, but the surgical group was treated with fewer medications than the non-surgical group. We believe this difference is mainly owing to the effect of the IOP-lowering surgery.
Although patients who eventually required IOP-lowering surgery received a similar number of injections compared to the non-surgical group, their IOP remained elevated. In contrast to NVI, which showed rapid regression and stabilized up to 3 months after injection, NVA in the surgical group showed less response to injection. Actually, NVA progressed regardless of treatment in the surgery group. Previous reports have shown various results for NVI and NVA regression after intraocular bevacizumab injection. Bhagat et al. [16] reported that NVA was significantly reduced up to 8 weeks after intravitreal or combined intravitreal and intracameral bevacizumab injections. Yazdani et al. [17] reported variable regression of NVA after intravitreal bevacizumab injections. However, Kotecha et al. [18] reported that NVI was rapidly resolved 1 week after intravitreal bevacizumab injection, but there was no significant reduction in NVA.
Seven eyes could be treated non-surgically in this study. IOP, NVI, and NVA rapidly decreased after intracameral bevacizumab and stabilized during the 12 months of follow-up. However, a single intracameral injection was not enough for IOP and new vessel control. These patients received 2.40 ± 1.72 injections, and five eyes (71%) required more than two injections. They also had additional PRP, and six eyes (86%) had complete PRP.
We identified the predictive factors for IOP-lowering surgery after intracameral bevacizumab in NVG patients by using multivariate analysis. Patients with higher initial IOP and advanced NVA grade tended to have IOP-lowering surgery. In addition, patients who underwent complete PRP had less frequent surgical treatments. Most of eyes in the non-surgical group (6 of 7 eyes, 86%) showed more frequent complete PRP and showed less NVA than the surgical group. This suggests that patients with early stage NVG responded better to intracameral bevacizumab injections and tended to require IOP-lowering surgery to a lesser extent. Nakano et al. [19] recently reported that NVG patients with retinal vein occlusion had a higher degree of angle closure and higher IOP, but NVG patients with PDR had lower IOP and better visual acuity than other underlying conditions. However, we did not find a significant relationship between preexisting ocular ischemic disease and prognostic factors for IOP-lowering surgery. Previous studies showed various results for corneal endothelial toxicity induced by intracameral bevacizumab injections. Hosny et al. [20] reported reduced mean endothelial cell count and hexagonality after intracameral bevacizumab, but there was no clinically significant corneal edema. Park et al. [21] reported that intracameral bevacizumab (up to 2.5 mg/0.1 mL) did not affect endothelial cell viability or morphology in rabbit cornea. In our study, we found no significant corneal endothelial damage in patients throughout the follow-up periods. Although corneal endothelial parameters tended to decrease after intracameral bevacizumab injection, the change was not statistically significant. Therefore, our results suggest intracameral bevacizumab injection is a relatively safe procedure for the treatment of NVG.
This study has several limitations. First, this is a retrospective study with a relatively small sample size (26 eyes). In addition, there was no control group (i.e., non-treated) or other route of injection (e.g., intravitreal or combined intracameral and intravitreal) against which to compare the effect of intracameral injection. Second, reduced abnormal new vessels and controlled IOP after treatment might be the effect of not only ICB but other treatment factors (e.g., PRP or IOP-lowering surgery). Actually, PRP was more completely performed in the nonsurgical group than the surgical group. However, these data suggest that ICB in NVG patients, especially in early stage NVG, can provide a temporary therapeutic window for the completion of PRP and therefore reduce the risk of IOP-lowering surgery. Third, this study measured NVI and NVA only via slit-lamp microscopy and gonioscopy. If we had evaluated the changes in new vessels with anterior segment fluorescein angiographic imaging and measured VEGF levels in the aqueous humor after treatment, we could have explained the effect of bevacizumab more objectively. Nevertheless, this is the first investigation of the efficacy and safety of intracameral bevacizumab injection in a Korean population at 1-year follow-up.
In conclusion, intracameral bevacizumab rapidly improved NVI, NVA, and IOP in NVG patients. However, the therapeutic effect was transient, and the majority of patients required multiple injections, additional laser treatment, and IOP-lowering surgery. The predictive factors for IOP-lowering surgery were baseline IOP, NVA grade, and complete PRP.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.