Influence of Biometric Variables on Refractive Outcomes after Cataract Surgery in Angle-closure Glaucoma Patients
Article information
Abstract
Purpose
To evaluate the influence of biometric variables on refractive outcomes after cataract surgery in angle-closure glaucoma (ACG) patients.
Methods
In this case-control study, 42 ACG patients, 40 open-angle glaucoma (OAG) patients, and 35 controls without glaucoma who had undergone conventional cataract surgery were enrolled consecutively. Electronic medical records, including preoperative biometric variables (keratometric diopter, axial length, anterior chamber depth, and lens thickness), the refractive change (RC), and the absolute value of refractive change (ARC) were reviewed.
Results
In the control and OAG patients, the anterior chamber depth was negatively correlated with the ARC (r = -0.344, p = 0.043 and r = -0.431, p = 0.006, respectively), whereas there was no correlation in the ACG patients. Lens thickness was positively correlated with the RC, but not with the ARC, in the control and OAG groups (r = 0.391, p = 0.020 and r = 0.501, p = 0.001, respectively). In contrast, lens thickness in the ACG group was not correlated with the RC but was positively correlated with the ARC (r = 0.331, p = 0.032).
Conclusions
In contrast with the anterior chamber depth, preoperatively measured lens thickness may be a useful predictor of the direction of the RC after cataract surgery in control and OAG patients. However, in ACG patients, a thicker lens was correlated with a larger RC, regardless of the direction of the shift (hyperopic or myopic).
After the introduction of phacoemulsification by Kelman [1] in the 1960s, surgeons have continually refined this technique to make it even safer and more effective. Recently, phacoemulsification with posterior chamber intraocular lens (IOL) implantation has become a standard procedure in cataract surgery. In order to meet a patient's postoperative expectations of improved visual outcomes after cataract extraction, one of the most important considerations is an accurate prediction of the refractive power. A critical factor in determining the postoperative refractive power is the postoperative position of the IOL (i.e., the effective lens position [ELP]). The ELP is predicted using IOL-power calculation formulas incorporating preoperatively determined biometric variables. To date, a number of IOL power calculation formulas have been proposed, some of which, including SRK/T, Hoffer Q, Holladay 1, and Haigis, have been widely employed in clinical settings. SRK/T, Hoffer Q, and Holladay 1 incorporate parameters including axial length and keratometric diopter [234], while the Haigis further uses the preoperatively determined anterior chamber depth to predict the ELP [5].
Currently, cataract surgery, which deepens the anterior chamber and reduces intraocular pressure (IOP) is one of the surgical treatment options for patients with angle-closure glaucoma (ACG) [67891011]. However, in ACG eyes, the anterior chamber depth is shallower and the lens is thicker compared with normal eyes. When the anterior chamber depth is altered after surgery, which in most cases results in deepening, an accurate ELP prediction is unlikely [121314]. Zonular weakness, which can accompany ACG, also influences the postoperative refractive outcome [1314].
In the present study, we investigated the change in the refractive error after cataract surgery in ACG patients relative to those in glaucoma-free cataract (control group) and open-angle glaucoma (OAG) patients. Additionally, we evaluated the influence of preoperative biometric variables, the primary determinants of postoperative ELP, on refractive outcome.
Materials and Methods
This was a retrospective case-control study. Review of patient data was approved by the institutional review board of Chungnam National University Hospital. The medical records of patients who had undergone phacoemulsification using posterior chamber IOL implantation by a single experienced surgeon (CSK) between January 1, 2010 and May 31, 2013 were reviewed. We consecutively enrolled three groups of cataract patients: ACG, OAG, and controls. Phacoemulsification was performed using a temporal clear corneal incision, and an aspheric acrylic IOL (AcrySof IQ IOL; Alcon, Fort Worth, TX, USA) was implanted into the capsular bag. Patients with other ocular diseases or previous ocular surgery were excluded. Cases with zonular dialysis, follow-up loss, or concurrent additional eye procedures, including vitrectomy and trabeculectomy, were also excluded. When both eyes met the inclusion criteria, one eye was randomly selected.
To meet the criteria for a diagnosis of ACG, patients had to have a narrow angle in which the trabecular meshwork was not visible upon gonioscopic examination of at least 270° in the primary position, a raised IOP of larger than 21 mmHg, glaucomatous optic neuropathy, and a reproducible glaucomatous visual-field defect according to the Swedish Interactive Threshold Algorithm of 24-2 perimetry (Humphrey Field Analyzer II; Carl Zeiss Meditec, Dublin, CA, USA). Glaucomatous optic neuropathy was characterized by focal or diffuse neuroretinal rim thinning, localized notching, or nerve fiber layer defects. A glaucomatous visual-field defect was defined by two of the following three criteria: the presence of a cluster of three points on a pattern deviation probability plot with a p-value less than 0.05, one of which has a p-value less than 0.01; a pattern standard deviation with a p-value less than 0.05; or glaucoma hemifield test results outside the normal limits.
The criteria for OAG diagnosis were a typical glaucomatous optic neuropathy, glaucomatous visual-field defect, and open angles on gonioscopy. All of the enrolled ACG and OAG patients had a treated IOP less than 22 mmHg with glaucoma medications, with the exception of miotics or cycloplegics, throughout the study period.
The normal control group was recruited from cataract patients who had visited the glaucoma clinic for a routine eye examination. None of the eyes showed any evidence of glaucomatous optic neuropathy or visual-field defects. Additionally, they had an IOP of less than 22 mmHg in the bilateral eye, no history of elevated IOP, and no family history of glaucoma.
The data collected at the final visit prior to cataract surgery included age, sex, laterality of the operated eye, past medical history, preoperative best-corrected visual acuity (BCVA), manifest refraction, IOP, number of anti-glaucoma medications, nuclear sclerosis grade of the cataract, biometric variables from a keratometric reading using an ophthalmokeratometer (Inami, Nagoya, Japan), axial length, anterior-chamber depth, crystalline lens thickness using an OcuScan (Alcon, Cleveland, TN, USA), and predicted postoperative refractive error. For comparative purposes, the visual acuities were converted to logarithm of the minimal angle of resolution (logMAR) equivalents and the refractions to spherical equivalents. The power of the implanted IOL was calculated using the Hoffer Q or SRK-T formula according to each patient's axial length; the former was used for short eyes (axial length ≤22.0 mm) and the latter for longer eyes (axial length >22.0 mm). The IOL power was determined between emmetropia and -1.0 diopter according to the surgeon's preference. Subsequently, the patients underwent cataract surgery as scheduled. Postoperative BCVA, manifest refraction, IOP, the number of anti-glaucoma medications, and the actual refractive error were determined and recorded a minimum of 2 months later (to avoid possible instability of the refractive error in the early postoperative period) a nd a maximum o f 4 months later. In this time, patients with early posterior capsular opacity or IOL instability, such as optic capture through a continuous curvilinear capsulotomy, were excluded. Finally, the refractive change (RC; postoperative actual refractive error minus preoperatively predicted refractive error) and the absolute value of refractive change (ARC) were calculated.
All statistical analyses were performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). The main outcome measures were the associations between the preoperative biometric variables and the RC and ARC, respectively, which were analyzed using univariate linear regression. The patients' demographics, including BCVA (logMAR), biometric variables, IOP, and values of refraction (spherical equivalent), were compared among the three groups using one-way analysis of variance (post-hoc multiple comparison) or Pearson chi-square test. A paired t-test was performed to compare the preoperative and postoperative IOP results, number of glaucoma medications, BCVA, and refractive error. A p-value less than 0.05 was taken to indicate statistical significance.
Results
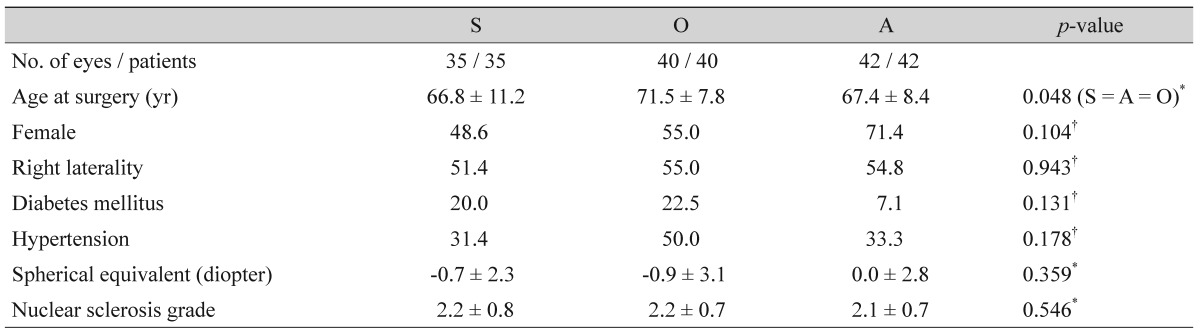
We analyzed the data for one eye of 42 ACG patients, 40 OAG patients, and 35 patients with a simple cataract who had undergone conventional cataract surgery. Table 1 summarizes the demographics of the three groups. Although the age at surgery displayed a statistically significant difference among the three groups, there were no differences between any two of the groups. The other demographics showed no statistically significant differences.
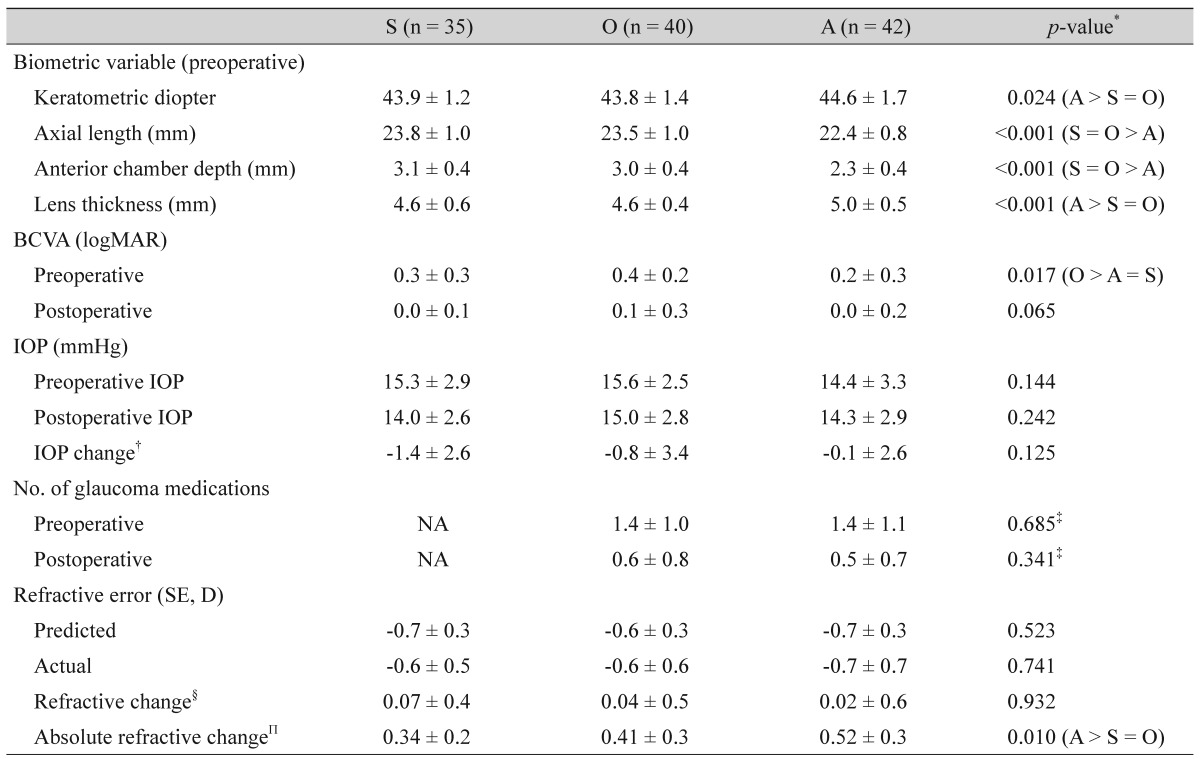
Of the biometric variables, the keratometric reading and lens thickness were higher and thicker, respectively, in the ACG group when compared with the control and OAG groups (keratometric reading, 44.6 vs. 43.9 and 43.8 diopters; lens thickness, 5.0 vs. 4.6 and 4.6 mm, respectively; p = 0.024, p < 0.001, respectively). In contrast, the axial length and anterior chamber depth were shorter and shallower, respectively, in the ACG group than in the control and OAG groups (axial length, 22.4 vs. 23.8 and 23.5 mm; anterior chamber depth, 2.3 vs. 3.1 and 3.0 mm, respectively, both p < 0.001). The preoperative BCVA was poorer in the OAG group compared with the ACG group (0.4 vs. 0.2 logMAR, p = 0.017), whereas postoperatively, there was no significant difference among the three groups (p = 0.065). There was no significant difference in the RC among the three groups. The ARC in the ACG group was greater than those in the control and OAG groups, and this was statistically significant (p = 0.010). There was no significant difference in the ARC between the control and OAG groups (Table 2). The preoperative and postoperative IOP did not differ in either of the glaucoma groups (p > 0.1 in both groups), whereas it was significantly decreased in the control group (15.3 vs. 14.0, p = 0.010). In contrast, the number of glaucoma medications in both glaucoma groups decreased postoperatively (p < 0.001 in both groups). The BCVA of all three groups was significantly improved by cataract surgery (p < 0.001 in all three groups).
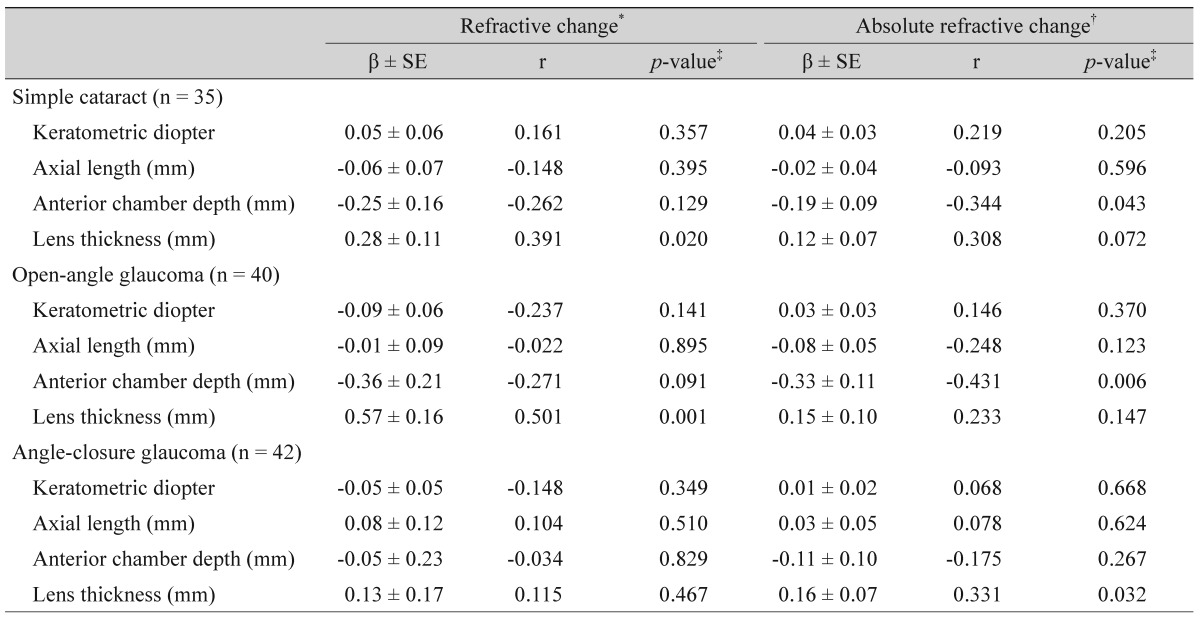
Univariate linear regression analysis indicated that in both the control and OAG groups, a shallower anterior chamber depth was associated with an increased ARC (correlation coefficient [r] = -0.344, p = 0.043 and r = -0.431, p = 0.006, respectively), and that as the lens thickened, the hyperopic shift became more significant (r = 0.391, p = 0.020 and r = 0.501, p = 0.001, respectively). In the ACG group, neither the anterior chamber depth nor the lens thickness influenced the RC, whereas the magnitude of the ARC significantly increased as the lens thickened (r = 0.331, p = 0.032) (Table 3). The other biometric variables, keratometric diopter and axial length, displayed no significant association with the RC or ARC in any of the groups.
In the combined scattergram of 75 control and OAG patients, as the lens thickened and the anterior chamber depth became shallower, a hyperopic shift was observed (r = 0.428, p < 0.001 and r = -0.234, p = 0.039, respectively) (Fig. 1A), and the ARC increased (r = 0.264, p = 0.019 and r = -0.394, p < 0.001, respectively) (Fig. 1B). However, in the multivariate regression analysis, only the lens thickness was associated with the hyperopic shift (regression coefficient [β] = 0.513, p < 0.001), and only the anterior chamber depth was related to the ARC (β = -0.281, p = 0.007). In contrast, as the lens thickened in the ACG group, there was a statistically significant increase in the ARC, whereas there was no significant correlation between the lens thickness and the RC with respect to either a hyperopic or myopic shift (β = 0.169, r = 0.331, p = 0.032). The anterior chamber depth displayed no significant correlation with the RC or ARC (Fig. 2A and 2B). The preoperative/postoperative IOP or IOP change was not associated with the RC or ARC in any of the three groups (all p > 0.1).

Scattergrams comparing the lens thickness (LT) and anterior chamber depth (ACD) with the refractive change (RC) and absolute refractive change (ARC) in the eyes of normal control subjects and open-angle glaucoma patients who underwent cataract extraction with intraocular lens implantation (n = 75). (A) Correlation between LT and RC (r = 0.428, p < 0.001) and between ACD and RC (r = -0.234, p = 0.039). Multivariate regression analysis formula, RC = 0.198 × ACD + 0.513 × LT - 2.908 (ACD, p = 0.257; LT, p < 0.001). (B) Correlation between LT and ARC (r = 0.264, p = 0.019) and between ACD and ARC (r = -0.394, p < 0.001). Multivariate regression analysis formula, RC = -0.281 × ACD - 0.029 × LT +1.374 (ACD, p = 0.007; LT, p = 0.726). r = correlation coefficient. *Postoperative actual refractive error minus preoperatively predicted refractive error; †Absolute value of refractive change.

Scattergrams comparing the lens thickness (LT) and anterior chamber depth (ACD) with the refractive change (RC) and absolute refractive change (ARC) in patients with angle-closure glaucoma who underwent cataract extraction with intraocular lens implantation (n = 42). (A) Correlation between LT and RC (r = 0.115, p = 0.467) and between ACD and RC (r = -0.034, p = 0.829). (B) Correlation between LT and ARC (β = 0.169, r = 0.331, p = 0.032) and between ACD and ARC (r = -0.175, p = 0.267). r = correlation coefficient; β= regression coefficient. *Postoperative actual refractive error minus preoperatively predicted refractive error; †Absolute value of refractive change.
Discussion
The postoperative refractive outcome is normally determined by preoperative biometry and the IOL power calculation formula. Among the currently popular third-generation IOL power calculation formulas, the SRK-T formula results in less refraction error over the whole axial length, particularly in long eyes (axial length ≥26 mm), while the Hoffer Q formula is clinically more accurate than the SRK/T formula in shorter eyes of less than 22.0 mm [1516]. In the present study, we used the target refractive power as calculated by these two formulas based on the axial length. The results of this study indicated that in the OAG and control groups, the RC tended to be hyperopic with a thicker lens. In contrast, we observed that as the lens thickened in the ACG group, the ARC increased, regardless of the direction of the RC (myopic or hyperopic). In the control and OAG groups, the ARC increased as the preoperative anterior chamber depth became shallower, as was similarly reported in previous studies [1718]. In contrast, in the ACG group, there was no apparent correlation between the RC or ARC and the preoperative anterior chamber depth. These results regarding the direction of the correlation (positive or negative) and statistical significance of the biometric variables in each group were sustained with a single formula: the SRK-T.
ACG eyes compared with normal and OAG eyes are characterized by a thicker lens and shallower anterior chamber and also differed in anatomical aspects, including zonular weakness and a crowded anterior segment [13192021]. A thicker crystalline lens (i.e., greater capsular volume) and zonular weakness may be responsible for postoperative IOL instability and shifts in the myopic and astigmatic refractive powers via IOL tilting, or shifts in the hyperopic refractive power via posterior shifts in the IOL [13142122]. In t he present study, the A RC was greatest in t he ACG group, which is associated with the thickness of the preoperatively measured lens. Kang et al. [22] used the SRK II formula to report that the ARC in ACG eyes was greater than that in normal eyes, and that the biometric variables (i.e., lens thickness, anterior c hamber d epth, and a xial length) were unrelated to the RC, which are consistent with our results. However, they did not investigate the correlation between biometric variables and the ARC.
The narrow anterior chamber in ACG patients is closely related to the thickness of the crystalline lens; with age, it becomes shallower as the lens thickens. A more anteriorly positioned crystalline lens was also associated with the shallower anterior chamber (anterior crowding) [12]. Indeed, in t he present study, we noted t hat w ith a t hicker crystalline lens, the anterior chamber depth was significantly shallower (r = -0.722, p < 0.001). In patients with a shallower anterior chamber due to a thicker crystalline lens, the ELP was more posterior than the preoperative prediction, because of the large capsular bag (posterior bulging) after cataract surgery; in turn, the RC was more hyperopic because of the posterior IOL shift in the capsular bag. In addition, a more anteriorly positioned IOL than the predicted ELP is observed occasionally in subjects with a shallower anterior chamber caused by anterior segment crowding, regardless of the lens thickness.
A more accurate postoperative refractive predictability may be achieved by measuring the actual ELP immediately following cataract extraction. A recent study that determined the ELP (the length between the cornea and the posterior lens capsule) using intraoperative anterior-segment optical coherence tomography demonstrated that a better prediction was obtained after capsular tension ring implantation following crystalline lens removal in cataract surgery [23]. In general, the intraoperative measurement of the lens capsule position can be expected to facilitate an improved ELP estimation in ACG patients with a large ARC and a difficult-to-predict hyperopic or myopic shift.
IOP lowering effects of cataract surgery have been reported, regardless of the presence of glaucoma [689]. Although a reduction of IOP after cataract surgery in the OAG and ACG patients was not significant in this study, the decrease in the number of glaucoma medications was statistically significant.
The present study had several limitations. One was its retrospective approach to the assessment of refractive and biometric changes, while a second was the small number of patients enrolled in each group. A third limitation was the use of applanation A-scan ultrasounds to determine the axial length, lens thickness, and anterior chamber depth, instead of non-contact optical biometry, including an IOLMaster (partial coherence interferometry), because the lens thickness could not be determined using an IOLMaster. The values of the axial length and anterior chamber depth using applanation A-scan ultrasounds are shorter than those using an IOLMaster [24]. However, both Giers and Epple [25] and Binkhorst [26] reported that biometric variables can be determined accurately by an experienced technician without using corneal depression. Indeed, in the present study, we used the results obtained by an experienced technician to minimize the error. A final limitation of the study was that certain postoperative biometric variables (i.e., anterior chamber depth, keratometric diopter, and axial length), were unavailable because of their retrospective nature. In this context, a future, prospective study evaluating a larger population of ACG patients is required.
In conclusion, when the SRK-T and Hoffer Q formulas are used to calculate IOL power, the anterior chamber depth and lens thickness should also be evaluated, as both can affect the direction and magnitude of the RC. Additionally, it should be kept in mind that: (1) in ACG patients, the RC direction is difficult to predict, (2) the ARC may be greater than that in the cataract only or OAG patients, and (3) as the lens thickens, the ARC tends to increase.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.