|
 |
| Korean J Ophthalmol > Volume 29(5); 2015 > Article |
Abstract
Purpose
To evaluate the long-term outcomes of intravitreal anti-vascular endothelial growth factor (VEGF) monotherapy for patients diagnosed with submacular hemorrhage secondary to exudative age-related macular degeneration.
Methods
This retrospective, observational study included 49 patients (49 eyes) who initially presented with submacular hemorrhage associated with exudative age-related macular degeneration and who were followed-up for at least 24 months. Only eyes that were treated with intravitreal anti-VEGF monotherapy were included in the study. Best-corrected visual acuity (BCVA) measurements obtained at diagnosis, six months, and the final visit were compared. The associations of BCVA at the final visit with baseline BCVA, BCVA at six months, symptom duration, hemorrhage extent, and central foveal thickness were also analyzed.
Results
Over the course of follow-up (mean, 32.1 ± 8.5 months), an average of 5.1 ± 2.2 anti-VEGF injections were administered. Recurrent hemorrhage was noted in 13 eyes (26.5%). The mean logarithm of the minimal angle of resolution BCVA at diagnosis, six months, and the final visit were 1.40 ± 0.52, 0.87 ± 0.64, and 1.03 ± 0.83, respectively. Both baseline BCVA (p = 0.012) and BCVA at six months (p < 0.001) were significantly associated with BCVA at the final visit.
Intravitreal anti-vascular endothelial growth factor (VEGF) is an effective treatment for exudative age-related macular degeneration (AMD) [1,2,3,4,5,6]. Recent studies have shown that the effect of intravitreal anti-VEGF is also demonstrated in eyes with submacular hemorrhage [7,8,9,10,11,12]. However, the previous studies that investigated anti-VEGF monotherapy efficacy in eyes with submacular hemorrhage only followed their patients for 12 months, despite the fact that outcome changes have been reported 2 to 3 years after therapy for submacular hemorrhages that occur secondarily to exudative AMD [13,14,15,16,17]. These prior studies mostly date to the era before anti-VEGF therapy. Little information is available regarding long-term outcomes after treatment with anti-VEGF therapy [17].
Recently, we reported on the efficacy of anti-VEGF monotherapy in 91 eyes with submacular hemorrhage after an initial presentation of exudative AMD [11]. In the present study, we report the extended long-term outcomes associated with treatment administered as part of the previous study. We also discuss the recurrence of hemorrhage and factors predictive of long-term visual prognosis.
This retrospective, observational case series was performed at a single center according to the tenets of the Declaration of Helsinki. The study was approved by the institutional review board of Kim's Eye Hospital.
The present study included patients from the same cohort and used similar inclusion and exclusion criteria as were used in our previous study [11]. A computerized search for patients who were newly diagnosed with exudative AMD from September 2009 to November 2012 at our institution was conducted. Fovea-involving subretinal hemorrhages extending over at least 50% of the lesion area or at least three disc areas were included. Additionally, only patients who exhibited initial visual acuity of 20 / 30 or worse and who were treated with intravitreal ranibizumab were included. Only newly diagnosed, treatment-naïve eyes were included. Patients who had completed two years or more of follow-up were included in the result analysis.
All subjects underwent a comprehensive ophthalmologic examination, including best-corrected visual acuity (BCVA) measurement, 90-diopter lens slit-lamp biomicroscopy, fundus photography, fluorescein angiography, and spectral domain optical coherence tomography (either Spectral OCT/SLO, OTI Ophthalmic Technologies, Ontario, Canada; or Spectralis, Heidelberg Engineering, Heidelberg, Germany). Indocyanine green angiography was performed using a confocal laser-scanning system (HRA-2, Heidelberg Engineering) at the discretion of each physician. The exclusion criteria included less than six months of follow-up, duration of symptoms longer than six months, severe media opacity, evidence of end-stage AMD such as central geographic atrophy or disciform scarring, evidence of a macroaneurysm, proliferative diabetic retinopathy, central retinal vascular occlusion, or history of intraocular surgery other than cataract surgery. Eyes that had undergone pneumatic displacement or photodynamic therapy, as well as eyes that had received intravitreal injections of tissue plasminogen activator or photodynamic therapy during the follow-up period, were also excluded. The number of eyes that underwent vitrectomy or cataract surgery during the follow-up period due to the development of severe vitreous hemorrhage was recorded; however, all data pertaining to these eyes were excluded from the analyses. If a submacular hemorrhage developed in both eyes, the eye that was affected first was included; thus, only one eye was included for each patient.
Visual acuities were converted to logarithm of minimal angle of resolution (logMAR). As recommended by Holla-day [18], "counting fingers" and "hand-motion" were converted to logMAR equivalents 2 and 3, respectively. The extent of hemorrhage was estimated using disc area as the basic unit. Central foveal thickness was defined as the distance between the internal limiting membrane and Bruch's membrane at the fovea and was manually measured using the calipers provided by an optical coherence tomography software program. We were concerned about the accuracy of hemorrhage-extent measurements exceeding 20 disc areas and central foveal thickness values exceeding 1,500 µm and therefore set these as threshold values. Lesions exceeding these measurements were recorded as 20 discs or 1,500 µm thickness, respectively. All extent-of-hemorrhage and central foveal thickness measurements were estimated by a single examiner (JHK).
The indocyanine green angiography results were analyzed by two independent examiners (JHK and YSC). Cases of exudative AMD were classified as typical exudative AMD or polypoidal choroidal vasculopathy (PCV) based on the indocyanine green findings. Cases exhibiting branching vascular networks and/or terminating polypoidal lesions were diagnosed as PCV. In some cases, the presence of late geographic hyperfluorescence on indocyanine green angiography [19] and/or the double-layer sign on optical coherence tomography [20,21] were observed in association with a branching vascular network on indocyanine green angiography. These cases were classified as PCV even if no definite polypoidal lesions were identified. All other cases were classified as typical exudative AMD. For cases in which a definite initial diagnosis was not possible using indocyanine green angiography, indocyanine green angiography images collected within six months after diagnosis were reviewed. Any disagreements were settled by discussion between the examiners.
Patients were initially treated with either ranibizumab (Lucentis; Genentech, San Francisco, CA, USA) or bevacizumab (Avastin, Genentech). As an initial treatment, 1 to 3 monthly intravitreal anti-VEGF injections were administered. Following initial treatment, patients were scheduled to visit the hospital once every 1 to 4 months based on status. Optical coherence tomography examinations were performed once every 1 to 6 months at the discretion of the clinician. Retreatment with intravitreal anti-VEGF usually occurred when intraretinal/subretinal fluid was present after the initial injections or when intraretinal/subretinal fluid or retinal/subretinal hemorrhage recurred and was accompanied by an increase in macular thickness. Some PCV cases were treated with photodynamic therapy at the discretion of the treating physician.
Similar to our prior study, we also analyzed values of central foveal thickness, extent of hemorrhage, and BCVA up to 12 months of follow-up. The BCVA in the period between 12 months of follow-up and the final visit was newly measured in the present study. The analyses are described in the following sections.
Baseline BCVA, BCVA at six months, BCVA at 12 months, and BCVA at the final visit were compared. Eyes with BCVA of 20 / 40 or better, between 20 / 400 and 20 / 40, and 20 / 400 or worse were classified into the fair vision group, moderate vision group, and poor vision group, respectively. The distributions of eyes into the three groups at six months and at the final visit were compared. The associations of BCVA at the final visit with baseline BCVA, BCVA at six months, symptom duration, hemorrhage extent, and central foveal thickness were analyzed.
Values for symptom duration, central foveal thickness, hemorrhage extent, and number of anti-VEGF injections were compared between the typical exudative AMD group and the PCV group. Additionally, baseline BCVA, BCVA at six months, BCVA at 12 months, and BCVA at the final visit were compared within each group.
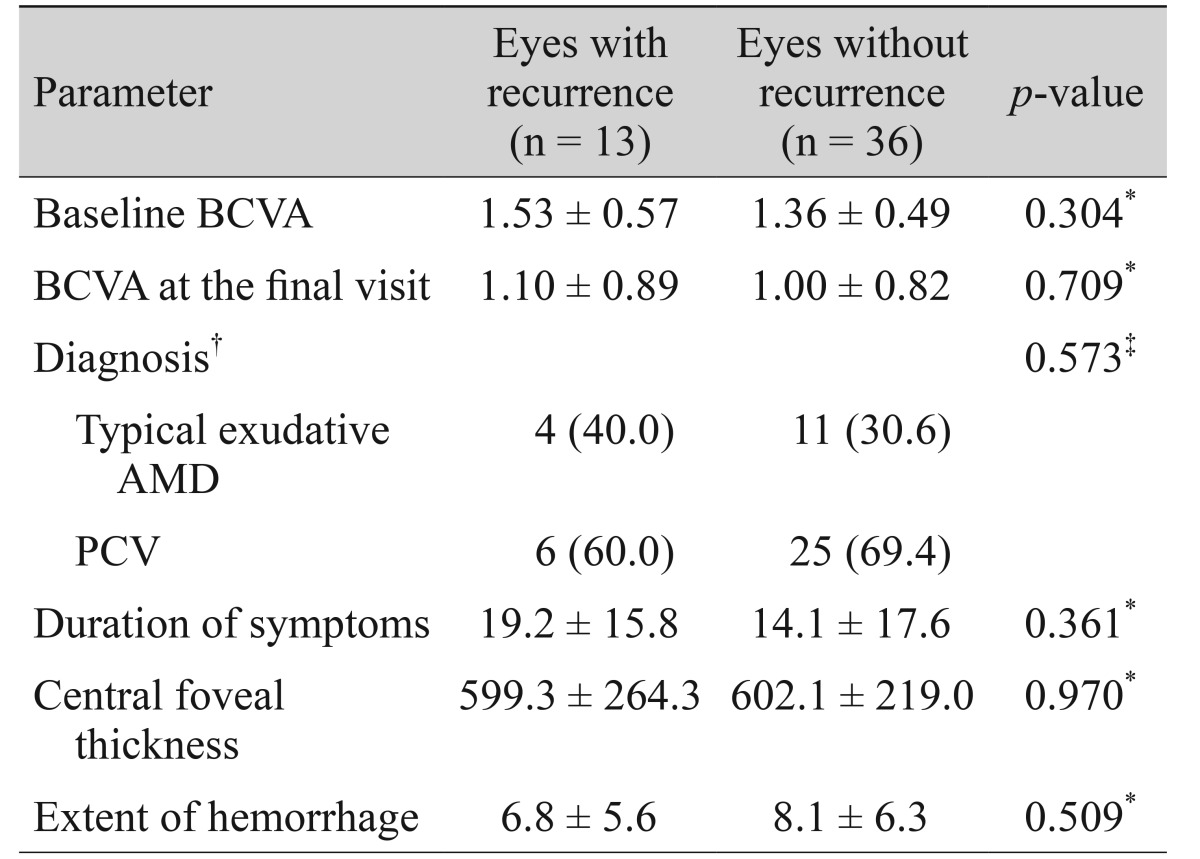
The number of eyes that experienced a recurrence of fovea-involving submacular hemorrhage of at least one disc area during the follow-up period was noted. Baseline BCVA and BCVA at the final visit were compared between eyes that did and did not experience recurrent hemorrhage. Baseline characteristics, including age, diagnosis, central foveal thickness, and hemorrhage extent, were compared between the groups.
The proportion of cases for each diagnosis (typical exudative AMD vs. PCV vs. unclassified), baseline BCVA, duration of symptoms, hemorrhage extent, and central foveal thickness were compared between the included eyes and the excluded eyes. The association between the number of anti-VEGF injections received throughout the entire follow-up period in patient with BCVA at the final visit and overall change in BCVA during the follow-up period was also analyzed.
Data are presented as mean ± standard deviation when applicable. Statistical analyses were performed with a commercially available software package (SPSS ver. 12.0; SPSS Inc., Chicago, IL, USA). Differences among various time points were analyzed using a repeated-measures analysis of variance, and individual comparisons were performed using Bonferroni's method. Differences between groups were analyzed using an independent-samples t-test or a chi-square test. Multivariable analysis was performed using a multiple stepwise linear regression model. Pearson correlation analysis was conducted to verify any associations between variables. A p-value <0.05 was considered significant.
Among the 159 patients that initially presented with submacular hemorrhage, 91 satisfied all eligibility criteria and were followed-up for six months or longer. The six-month clinical outcomes of these patients were presented in a previous study [11]. Among the 91 patients, 55 completed two or more years of follow-up. Two of these 55 patients underwent cataract surgery, two underwent vitrectomy, and two underwent photodynamic therapy between the first six months post-therapy and the final visit. Finally, 49 of the 91 enrolled patients (53.8%) were included in the analyses for this manuscript (Table 1). Thirty of the patients (61.2%) were men and 19 (38.8%) were women. The mean age was 68.6 ± 8.6 years (range, 51 to 86 years), and the mean symptom duration was 15.5 ± 17.1 days (range, 1 to 90 days). The mean BCVA was 1.40 ± 0.52 (Snellen equivalent, 20 / 502; range, counting fingers to 20 / 40). The mean hemorrhage extent was 7.7 ± 6.1 disc areas (range, 3 to 20), and the mean central foveal thickness was 601.4 ± 228.9 µm (range, 331 to 1,300 µm). The mean follow-up period was 32.1 ± 8.5 months from diagnosis. During this period, patients were treated with 5.1 ± 2.2 (range, 1 to 11) intravitreal anti-VEGF injections. A mean of 3.7 ± 1.2 injections were administered during the first 12 months, and a mean of 1.4 ± 1.4 injections were administered during the period extending from the 12-month follow-up to the final visit. Thirty-five eyes were treated with ranibizumab only, another 14 eyes were treated with both ranibizumab and bevacizumab, and the single remaining eye was treated with bevacizumab only. All the included eyes received at least one intravitreal anti-VEGF injection during the first 12 months. Thirty-one eyes (63.3%) received additional treatment at some point between the 12-month follow-up visit and the final follow-up visit.
For the 42 excluded eyes, measurement values were: baseline BCVA = 1.37 ± 0.60 (Snellen equivalent, 20 / 468; range, hand motion to 20 / 30), symptom duration = 41.8 ± 51.9 days, hemorrhage extent = 7.8 ± 5.2 disc areas, and central foveal thickness = 620.3 ± 274.1 µm. The group of excluded eyes had a significantly longer mean symptom duration than the included eyes (p = 0.003). None of the differences in baseline BCVA, hemorrhage extent, and central foveal thickness between the two groups were significant (p = 0.744, p = 0.827, and p = 0.909, respectively).
Fig. 1 shows a representative case of long-term change in the macular microstructure of an eye with submacular hemorrhage. The mean BCVA values at baseline, six months post-diagnosis, 12 months post-diagnosis, and at the final visit were 1.40 ± 0.52 (Snellen equivalent, 20 / 502), 0.87 ± 0.64 (Snellen equivalent, 20 / 148), 0.88 ± 0.68 (Snellen equivalent, 20 / 151), and 1.03 ± 0.83 (Snellen equivalent, 20 / 214), respectively (Fig. 2A). BCVA values differed significantly among the four time points (p < 0.001). The mean BCVA at the final visit showed significant improvement compared to the baseline value (p = 0.012), whereas the differences between the BCVA values at six months and at 12 months were not significantly different from BCVA at the final visit (p = 0.156 and p = 0.113, respectively). Compared to baseline values, a BCVA improvement of three lines or more was noted in 28 eyes (57.1%) at the final visit. A deterioration of three or more lines was noted in nine eyes (18.4%). The remaining 12 eyes (24.5%) exhibited stable BCVA throughout the follow-up period.
Compared to the six-month values, a BCVA improvement of three or more lines was noted in seven eyes (14.3%) at the final visit. A deterioration of three or more lines was noted in 15 eyes (30.6%). The remaining 27 eyes (55.1%) exhibited stable BCVA during the follow-up period. The number of anti-VEGF injections was not associated with BCVA at the final visit (p = 0.470) or the degree of change in BCVA during the follow-up period (p = 0.151).
At six months, the numbers of eyes included in the fair vision group (BCVA 20 / 40 or better), moderate vision group (BCVA from 20 / 400 to 20 / 40), and poor vision group (20 / 400 or worse) were 15 (30.6%), 20 (40.8%), and 14 (28.6%), respectively. The mean number of eyes in these groups at the final visit were 15 (30.6%), 16 (32.7%), and 18 (36.7%), respectively. The distribution of eyes among the three groups was not different between the six-month and final visits (p = 0.766). When classified based on BCVA at six months, six eyes (40.0%) in the fair vision group required additional treatment at some point between 12 months and the final follow-up. The number of eyes that received additional treatment was 15 (75.0%) in the moderate vision group and 10 (71.4%) in the poor vision group. The proportion of eyes that required additional treatment during the aforementioned period was not significantly different among the three groups (p = 0.065). In the fair vision group, only one eye (6.7%) experienced three lines of deterioration in BCVA between the six-month visit and the final follow-up. In the remaining 14 eyes (93.3%), BCVA remained stable throughout the entire follow-up period.
Indocyanine green angiography results obtained at the time of diagnosis or within six months after diagnosis were available for all included eyes. Among them, 15 (30.6%) and 31 eyes (63.3%) were ultimately diagnosed with typical exudative AMD and PCV, respectively. A definite diagnosis was not possible in the remaining three cases (6.1%) because a thick subretinal hemorrhage precluded obtainment of reliable indocyanine green angiography images of the lesion.
In the typical exudative AMD group, symptom duration, central foveal thickness, hemorrhage extent, and number of anti-VEGF injections were 17.8 ± 15.7 days, 543.5 ± 213.4 µm, 6.2 ± 5.9 disc areas, and 5.5 ± 2.5, respectively. In the PCV group, the values were 13.9 ± 19.1 days, 637.5 ± 239.9 µm, 8.3 ± 5.9 disc areas, and 4.9 ± 2.1, respectively. There were no significant differences between the two groups for these four parameters (p = 0.503, p = 0.209, p = 0.264, and p = 0.406, respectively) (Table 2). In the typical exudative AMD group, the mean baseline BCVA, at six months, at 12 months, and at the final visit were 1.49 ± 0.49, 0.94 ± 0.65, 0.97 ± 0.77, and 1.13 ± 0.87, respectively (Fig. 2B). In the PCV group, the values were 1.36 ± 0.54, 0.80 ± 0.64, 0.77 ± 0.64, and 0.87 ± 0.86, respectively. In unclassified eyes, the values were 1.43 ± 0.56, 0.19 ± 0.67, 1.20 ± 0.62, and 1.28 ± 0.53, respectively.
Compared with the baseline value, BCVA at six months was significantly improved for both the typical exudative AMD (p = 0.031) and PCV (p = 0.001) groups. For the PCV group, the changes in BCVA at 12 months (p < 0.001) and at final follow-up (p = 0.007) compared to baseline were also statistically significant, but these same comparisons were not significant in the AMD group (12 months, p = 0.177; final follow-up, p = 0.145).
Both baseline BCVA and BCVA at six months were significantly associated with BCVA at the final visit (p = 0.012 and p < 0.001, respectively) (Table 3). No other factors, including symptom duration, hemorrhage extent, and central foveal thickness, were associated with BCVA at the final visit (p = 0.841, p = 0.083, and p = 0.121, respectively) (Table 2). In multivariate analysis, BCVA at six months was found to be the factor most strongly associated with BCVA at the final visit (p < 0.001) (Table 2).
Fifteen recurrences of fovea-involving submacular hemorrhage occurred in 13 eyes (26.5%) during the follow-up period (Fig. 3). Two of these eyes experienced multiple recurrences. On average, hemorrhages reoccurred by 15.0 ± 11.1 months (range, 4 to 42) after diagnosis. Four (30.8%) and six eyes (46.2%) were diagnosed with typical exudative AMD and PCV, respectively. The remaining three eyes (23.1%) were unclassified. The results of comparisons between eyes with and without hemorrhage recurrence are summarized in Table 4. There was no difference in baseline BCVA (p = 0.304), BCVA at the final visit (p = 0.709), diagnosis (p = 0.573), symptom duration (p = 0.361), central foveal thickness (p = 0.970), or hemorrhage extent (p = 0.509) between eyes with or without hemorrhage recurrence.
In this study, visual acuity in the majority of eyes that initially presented with submacular hemorrhage had improved or remained relatively stable between six months after diagnosis and the final visit (mean, 32.1 months). As a result, BCVA values at the final visit were still significantly improved compared to baseline. This result suggests that, although the development of submacular hemorrhage induces retinal damage, any remaining retinal function can be maintained long-term through continuous anti-VEGF therapy.
Notably, the number of injections received between 12 months post-diagnosis and the final visit was markedly lower than the number of injections received during the first 12 months after diagnosis. Only 63.3% of patients received additional injections during this period. The mean number of injections after 12 months was far less than that administered during the 12- to 24-month period in prior controlled clinical trials [3,4], despite our use of a longer time interval between the 12-month follow-up point and the final visit (20.1 months). The lower injection frequency after 12 months may reflect several factors. To facilitate the early detection and prompt treatment of recurrent exudation in patients with exudative AMD, it is generally recommended to perform monthly follow-ups, including monthly OCT examinations [3,22]. In the present study, OCT examination was not routinely performed during every visit, and the follow-up period varied from 1 to 4 months at the discretion of the treating physician. Thus, recurrent exudate may not have been detected promptly. Moreover, the re-accumulation of small amounts of intraretinal/subretinal fluid may have been missed. Some patients, particularly those with very low visual acuity, refused additional treatment without a guarantee that their vision would subsequently improve. Similarly, treating physicians decided not to administer additional treatments in some cases when no definite benefit was anticipated. Regardless of these possible explanations, however, the findings in patients with relatively good visual acuity (20 / 40 or better) at six months after diagnosis are noteworthy. In this group, almost all the eyes maintained relatively stable BCVA, although only 40% of them required additional treatment. This particular finding requires further explanation. We postulate that relatively large, active vascular lesions, such as major polyps, may contribute to frequent re-accumulation of intraretinal/subretinal fluid that may have ruptured. Although other vascular abnormalities persisted, the activity of the entire exudative AMD lesion may have decreased after the rupture of a major vascular lesion and thus reduced the frequency of recurrent exudation.
In a study by Hwang et al. [17] that evaluated the incidence of recurrent submacular hemorrhage in exudative AMD, the recurrence rate was 51.1% during the follow-up period (mean, 36.8 months). That rate is much higher than the rate reported in the present study (26.5%). A direct comparison between this study and that of Hwang et al. [17] may not be appropriate because they evaluated various treatment modalities, including photodynamic therapy, intravitreal anti-VEGF, pneumatic displacement, and vitrectomy, whereas all the patients in the present study were treated with only intravitreal anti-VEGF monotherapy. In Hwang et al. [17], the use of intravitreal anti-VEGF was associated with a reduced risk of recurrent hemorrhage. The relatively low recurrence rate found in the present study may also suggest a potential role of anti-VEGF monotherapy in preventing recurrent hemorrhage. Most hemorrhage recurrences developed within two years of diagnosis. However, some recurrences occurred after more than three years, suggesting that a lack of recurrence over this relatively long-term period may not guarantee complete stabilization of the lesion.
We also found a strong association between BCVA at six months and BCVA at the final visit. BCVA at six months was more closely associated with BCVA at the final visit than with baseline BCVA. A subretinal hemorrhage typically resolves or at least decreases markedly in size during the first six months [7,8,11]. Because a hemorrhage can block the visual stimulus, BCVA at six months rather than baseline BCVA may more accurately reflect underlying retinal function. In approximately two-thirds of eyes, visual acuity at six months was relatively unchanged from baseline or even improved over the course of a mean 32.1 months of follow-up. As a result, BCVA at the final visit had not significantly decreased compared to the values at six months post-diagnosis. This suggests that the sixmonth visual acuity measurement can be used as a reference value in any discussion with patients regarding long-term visual prognosis.
We also found that typical exudative AMD and PCV exhibited different visual outcomes. More specifically, a favorable long-term visual outcome was achieved only in the PCV group. PCV is a distinct entity from typical exudative AMD [23], and the incidence of PCV is generally higher in Asian populations than in European populations [24,25]. Additionally, hemorrhage is a frequent finding in PCV cases [26,27]. The majority of our patients were diagnosed with PCV. We believe that this high frequency is a result of the two aforementioned characteristics. While anti-VEGF monotherapy has been considered the most effective first-line therapy for typical exudative AMD [1,6], its efficacy in PCV is still a matter of controversy [28]. Although anti-VEGF therapy has been found to be effective in previous studies [29,30], its efficacy is generally inferior to that of photodynamic therapy for polyp regression [31]. For this reason, some experts recommend photodynamic therapy rather than anti-VEGF monotherapy as first-line therapy in PCV [28]. Despite this controversy, the treatment outcomes of anti-VEGF monotherapy in our patients with PCV were encouraging. As discussed above, it is possible that this favorable outcome may be partially in response to the rupture of a major polyp after a hemorrhage. In contrast, the outcomes of typical exudative AMD were less favorable. Although marked improvement in BCVA was noted during the first six months, deterioration of visual acuity typically occurred thereafter. As a result, BCVA values at 12 months and later were not different from those at baseline. We think that the symptom duration may have had an influence. Although statistical significance was not reached, symptom duration in the typical exudative AMD group was relatively longer than that of the PCV group, suggesting more accumulated damage to the retina in the AMD group. Another reason may be a possible difference in the degree of retinal degeneration between typical exudative AMD and PCV patients. It is well known that age-related degenerative changes in the retina, including drusen and pseudodrusen, are more markedly present in typical exudative AMD cases than in PCV cases [32,33]. Additionally, the choroid of eyes with typical exudative AMD is generally thinner than that of eyes with PCV [34]. We think that retinas with pre-existing degeneration may be more vulnerable to damage resulting from the development of subretinal hemorrhage. Furthermore, a thin choroid may not provide sufficient perfusion to the retina. Although testing these hypotheses is beyond the scope of this study, the less favorable outcomes associated with typical exudative AMD suggest the need for other treatments (e.g., pneumatic displacement). Further studies are needed to establish the appropriate treatment method to improve long-term outcomes of submacular hemorrhages that occur secondary to typical exudative AMD.
This study has several limitations. First, it was a retrospective study and included only a small number of patients who exhibited submacular hemorrhage as an initial presentation. Second, there was no common treatment protocol or follow-up/optical coherence tomography examination schedule. Thus, differences in treatment decisions among clinicians may have influenced the study results. Because optical coherence tomography, fluorescein angiography, and indocyanine green angiography were not routinely performed during the follow-up period, the longterm anatomical outcomes were not analyzed.
In conclusion, relatively stable long-term visual outcome can be achieved for the majority of eyes exhibiting submacular hemorrhage as a secondary symptom to exudative AMD when treated with intravitreal anti-VEGF monotherapy, despite recurrence of exudation and/or submacular hemorrhage. BCVA at six months was found to be a useful clinical index predictive of long-term visual prognosis.
Conflicts of interest
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-1431.


2. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006;113:363-372.e5.


3. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007;143:566-583.


4. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-1398.



5. Kwon YH, Lee DK, Kim HE, Kwon OW. Predictive findings of visual outcome in spectral domain optical coherence tomography after ranibizumab treatment in age-related macular degeneration. Korean J Ophthalmol 2014;28:386-392.



6. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432-1444.


7. Cho HJ, Koh KM, Kim HS, et al. Anti-vascular endothelial growth factor monotherapy in the treatment of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol 2013;156:524-531.e1.


8. Iacono P, Parodi MB, Introini U, et al. Intravitreal ranibizumab for choroidal neovascularization with large submacular hemorrhage in age-related macular degeneration. Retina 2014;34:281-287.


9. Shienbaum G, Garcia Filho CA, Flynn HW Jr, et al. Management of submacular hemorrhage secondary to neovascular age-related macular degeneration with anti-vascular endothelial growth factor monotherapy. Am J Ophthalmol 2013;155:1009-1013.


10. Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol 2007;144:886-892.


11. Kim JH, Chang YS, Kim JW, et al. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology 2014;121:926-935.


12. McKibbin M, Papastefanou V, Matthews B, et al. Ranibizumab monotherapy for sub-foveal haemorrhage secondary to choroidal neovascularisation in age-related macular degeneration. Eye (Lond) 2010;24:994-998.


13. Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 1996;16:183-189.


14. Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology 2004;111:1993-2006.



15. Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica 1999;213:97-102.


16. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol 1990;109:33-37.


17. Hwang JU, Yang SJ, Yoon YH, et al. Recurrent submacular hemorrhage in patients with neovascular age-related macular degeneration. Retina 2012;32:652-657.


19. Kang SW, Chung SE, Shin WJ, Lee JH. Polypoidal choroidal vasculopathy and late geographic hyperfluorescence on indocyanine green angiography. Br J Ophthalmol 2009;93:759-764.


20. Kim JH, Kang SW, Kim TH, et al. Structure of polypoidal choroidal vasculopathy studied by colocalization between tomographic and angiographic lesions. Am J Ophthalmol 2013;156:974-980.e2.


21. Sato T, Kishi S, Watanabe G, et al. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina 2007;27:589-594.


22. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-1908.



23. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990;10:1-8.


24. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol 2004;49:25-37.


25. Coscas G, Yamashiro K, Coscas F, et al. Comparison of exudative age-related macular degeneration subtypes in Japanese and French Patients: multicenter diagnosis with multimodal imaging. Am J Ophthalmol 2014;158:309-318.e2.


26. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 2003;121:1392-1396.


27. Byeon SH, Lee SC, Oh HS, et al. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol 2008;52:57-62.


28. Koh AH. Expert PCV Panel. Chen LJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina 2013;33:686-716.


29. Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol 2013;156:644-651.


30. Lee SY, Kim JG, Joe SG, et al. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol 2008;22:92-99.



31. Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012;32:1453-1464.


32. Yoneyama S, Sakurada Y, Mabuchi F, et al. Genetic and clinical factors associated with reticular pseudodrusen in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2014;252:1435-1441.


Fig. 1
Fundus photography and optical coherence tomography findings of an eye with submacular hemorrhage secondary to polypoidal choroidal vasculopathy. At the time of diagnosis, visual acuity was measured as 20 / 100 (A,B). At 6 months, the hemorrhage had resolved completely, and visual acuity had improved to 20 / 25 (C,D). The eye was treated with 5 ranibizumab injections during the 28-month follow-up period. At 28 months, visual acuity was maintained at 20 / 25 (E,F).

Fig. 2
Changes in the mean logarithm of minimal angle of resolution (logMAR) best-corrected visual acuity (BCVA) among eyes that received anti-vascular endothelial growth factor monotherapy for submacular hemorrhage secondary to exudative age-related macular degeneration, according to the follow-up period. (A) In all 39 eyes, BCVA at the final visit was significantly better than baseline BCVA (p = 0.012). The difference between BCVA at the final visit and BCVA at six months or 12 months was not significant (p = 0.156 and 0.113, respectively). (B) Changes in values when the patients were divided into two groups according to diagnosis. Solid line (closed circles) indicates eyes diagnosed with typical exudative age-related macular degeneration (n = 15); dashed line (closed squares) indicates eyes diagnosed with polypoidal choroidal vasculopathy (n = 31).
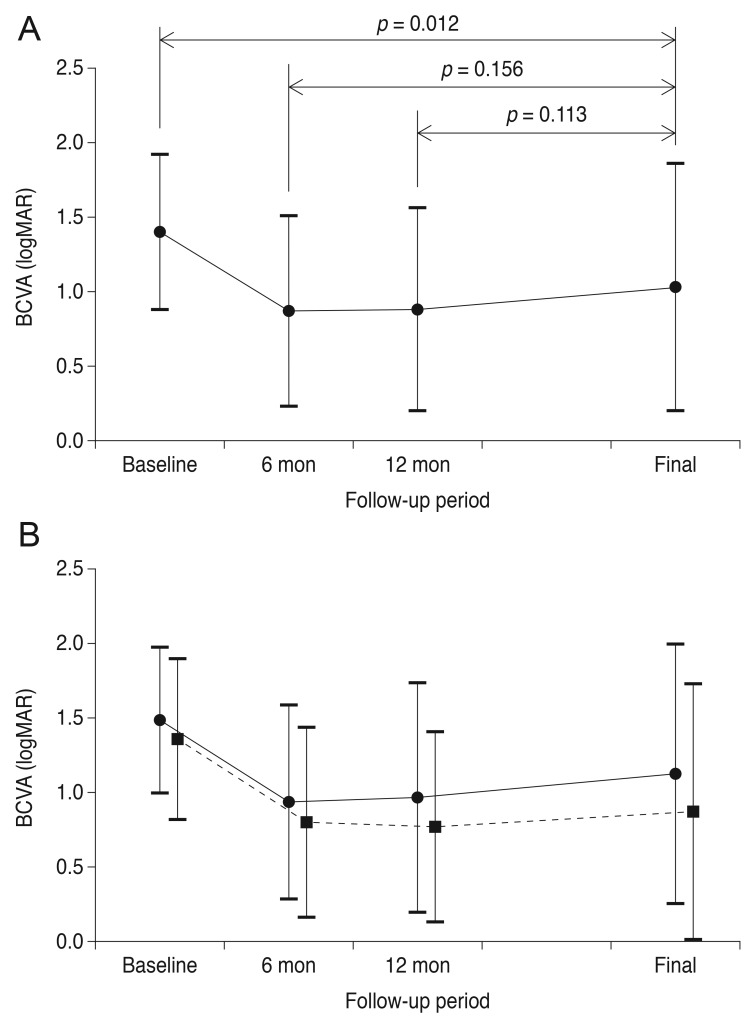
Fig. 3
A timetable showing the timing of recurrences of fovea-involving submacular hemorrhage according to the follow-up period. Inverted triangles indicate the first recurrence. Asterisks indicate the second recurrence. Fifteen recurrences were noted in 13 eyes. Two eyes experienced two episodes of recurrence.
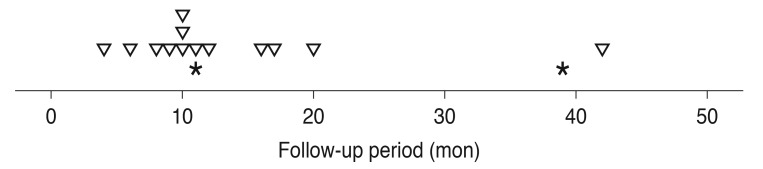
Table 1
Baseline characteristics of patients with exudative AMD with submacular hemorrhage as an initial presentation (n = 49)

Table 3
The associations of BCVA at the final visit with baseline BCVA, BCVA at six months, symptom duration, hemorrhage extent, and central foveal thickness
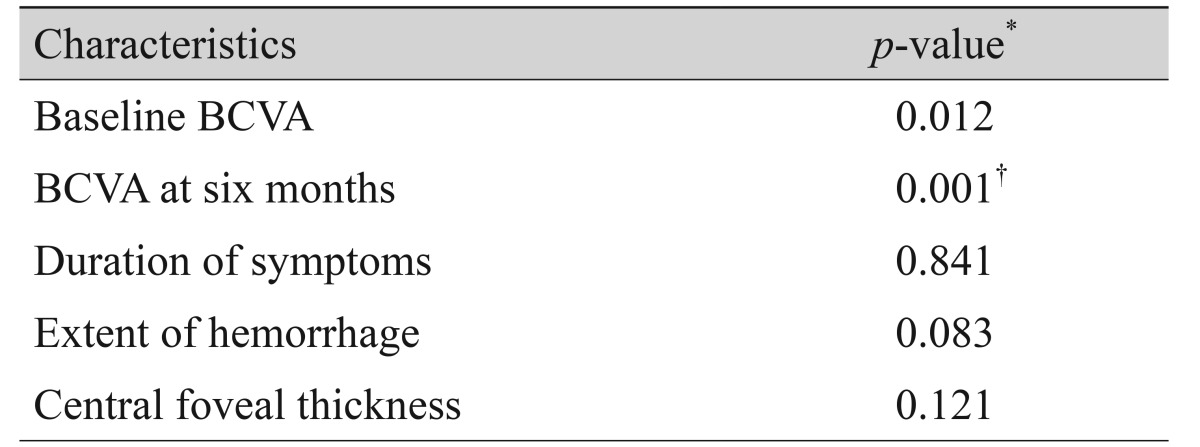
Table 4
Comparisons of parameters between eyes with and without recurring fovea-involving submacular hemorrhage
Values are presented as mean ± standard deviation or number (%).
BCVA = best-corrected visual acuity; AMD = age-related macular degeneration; PCV = polypoidal choroidal vasculopathy.
*Statistics were analyzed using independent samples t-test; †Analyses were performed for 46 eyes in which an accurate indocyanine-green angiography-based classification was possible; ‡Statistics were analyzed using chi-square test.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print