 |
 |
| Korean J Ophthalmol > Volume 29(5); 2015 > Article |
Abstract
Purpose
The incidence and clinical correlation of MALT1 translocation and chromosomal numerical aberrations in Korean patients with ocular adnexal mucosa associated lymphoid tissue (MALT) lymphoma have not yet been reported. We investigated the incidence and clinicopathologic relationship of these chromosomal aberrations in ocular adnexal MALT lymphomas in a Korean population.
Methods
Thirty ocular adnexal MALT lymphomas were investigated for the t(11;18) API2-MALT1, t(14;18) IgH-MALT1 translocations and chromosomes 3 and 18 aneuploidies using fluorescence in situ hybridization. Patient medical records were reviewed retrospectively for information on demographics and clinical characteristics, including treatment response.
Results
The MALT1 gene rearrangement was found in one out of 30 cases. The t(14;18) IgH-MALT1 translocation was demonstrated in only one case (3.3%), and the t(11;18) API2-MALT1 translocation was not found in any of the cases. Trisomy 3 was observed in three ocular adnexal MALT lymphomas (10.0%), and five cases showed trisomy 18 (16.7%). Translocation positive cases also showed trisomy 18. One case of tumor relapse showed trisomy 18 only in the recurrent biopsies. There were no statistically significant correlations between chromosomal aberrations and clinical characteristics and treatment responses.
Conclusions
Translocations involving the MALT1 gene are not common in Korean ocular adnexal MALT lymphomas. The t(14;18) translocation was detected in only one out of 30 cases, and the t(11;18) translocation was not found at all. Furthermore, the chromosomal aberrations found in this study had no prognostic implications.
Extra-nodal marginal zone lymphoma is the most frequent lymphoma subtype found in the orbit and ocular adnexa [1,2,3], and the incidence of non-Hodgkin's lymphoma of the ocular adnexa has steadily increased, especially in Asian-Pacific Islanders [4]. Extranodal marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) constitutes three-fourths of lymphoproliferative disease of the ocular adnexa in Korea [5], a higher proportion than that found in Western countries. Although the clinical course of most marginal zone lymphoma of MALT types is indolent, the pathogenesis and prognostic factors of this disease are largely unknown. However, it is generally thought that both chronic antigenic stimulation of infectious agents and acquired genetic or epigenetic alterations are involved [6,7,8]. Several recurrent structural and numerical chromosomal aberrations have been described in MALT lymphomas, but their incidence is quite variable depending on their primary site and geographical location [9,10].
The known balanced translocations in MALT lymphomas include t(11;18)(q21;q21) API2-MALT1, t(14;18)(q32;q21) IgH-MALT1, t(1;14)(p22;q32) BCL10-IgH, and t(3;14) (p14.1;q32) FOXP1-IgH [11]. Among these four translocations, t(11;18)(q21;q21) harboring the API2-MALT1 fusion is the most common chromosomal aberration reported in MALT lymphoma, and several reports have found that translocation-positive groups are associated with Helicobacter pylori (H. pylori) eradication failure, local aggressiveness and advanced clinical stage [12,13]. Another translocation involving MALT1, t(14;18)(q32;q21) IGH-MALT1, which is frequent in pulmonary or ocular type MALT lymphomas, is rarely observed in gastric MALT lymphomas [11,14]. Recently, numerical abnormalities of chromosomes 3, 7, 12, and 18 have been reported to be specific to marginal zone lymphoma of MALT types [15,16].
Ocular adnexal MALT lymphomas have not been studied genetically-except in a few studies with conflicting results-mainly because material for diagnostic biopsies is scarce and fresh tissue is not readily available [17,18,19].
To date, the incidence of translocation involving MALT1 in ocular adnexal MALT lymphoma has not been studied in the Korean population in spite of the relatively high prevalence of ocular adnexal MALT lymphoma compared to Western countries. Furthermore, the clinicopathologic relationship of these genetic abnormalities in ocular adnexal MALT lymphoma is not well-known.
In this study, we used two-color interphase fluorescence in situ hybridization (FISH) to determine the incidence of chromosomal aberrations involving MALT1 and numerical aberrations of chromosomes 3 or 18 in a cohort of ocular adnexal MALT lymphomas from Korean patients. We further analyzed the clinicopathologic relationship of these chromosomal aberrations to determine their prognostic relevance.
We collected 30 formalin-fixed, paraffin-embedded tissues of primary ocular adnexal MALT lymphomas, including one case of tumor recurrence, between 2002 and 2005 at Seoul Metropolitan Government-Seoul National University Boramae Medical Center and Seoul National University Hospital. Tissues were obtained from the orbit, lacrimal gland or conjunctiva of patients. Cases were reviewed and confirmed by hematopathologist (YAK) based on the criteria of the World Health Organization classification of tumors of hematopoietic and lymphoid tissues [20]. The relevant clinical information was taken from the medical records. This study was approved by the institutional review board of Seoul Metropolitan Government-Seoul National University Boramae Medical Center.
FISH analysis was used to investigate genetic aberrations such as MALT1 rearrangements and numerical aberrations of chromosomes 3 and 18. MALT1 rearrangements were detected using two-color interphase FISH and numerical aberrations were detected using one-color FISH.
Two-color interphase FISH for the detection of MALT1 translocation was performed on sections from paraffin blocks, as previously described [21]. Briefly, 4-µm-thick sections were deparaffinized, dehydrated, immersed in 0.2N HCl, boiled in a microwave in citrate buffer (pH 6.0), and incubated in 1M NaSCN for 35 minutes at 80℃. Sections were then immersed in pepsin solution, and tissues were fixed in 10% neutral-buffered formalin. The probe mixture was applied to the slides, which were then incubated in a humidified atmosphere with Hybrite (Vysis, Downers Grove, IL, USA) at 73℃ for 5 minutes to simultaneously denature the probe and target DNA and subsequently at 37℃ for 19 hours for hybridization. The slides were then immersed in 0.4× SSC/0.3% NP-40 for 2 minutes at room temperature, followed by 2× SSC/0.1% NP-40 for 5 minutes at 73℃. The nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) and anti-fade compound (p-phenylenediamine). FISH signals for each locus-specific FISH probe were assessed under an Olympus BX51TRF microscope (Olympus, Tokyo, Japan) equipped with a triple-pass filter (DAPI/Green/Orange; Vysis).
To determine the specific type of MALT1 translocation, FISH analysis was performed in two steps. The first step was to detect any MALT1 translocation using LSI MALT1 dual color break-apart probe (Vysis). Interphase nuclei of normal cells showed two yellow fusion signals (red+green). Rearrangement was defined as any splitting, i.e., red and green signal, on nuclei. To clarify the specific counterpart of MALT1 translocation, two fusion translocation probes were used in second step FISH. LSI API2/MALT1 dual color, dual fusion probe (Vysis) to detect t(11;18) (q21;q21) API2-MALT1 translocation and LSI IgH/MALT1 dual color, dual fusion probe (Vysis) to detect t(14;18) (q32;q21) IgH-MALT1 translocation. Interphase nuclei of normal cells showed two green signals for API2 or IgH and two red signals for MALT1. The presence of a translocation produced any one yellow fusion signals per nuclei.
To assess numerical aberrations, one-color FISH using centromeric repetitive α-satellite DNA probes specific for chromosomes 3 and 18 (Oncor, Gaithersburg, MD, USA) was performed. Interphase nuclei of normal cells showed two red signals respresenting two copies of chromosomes. Numerical gains of chromosomes, such as trisomy or polysomy, were defined as increased red signals on nuclei.
FISH signals were counted by previously described guidelines in the literature to reduce false positives and false negatives [22]. These guidelines are as follows: (1) only 'intact' (=spherical), non-overlapping nuclei with a clear counterstain were counted; (2) nuclei with paired spots (split spots) were not counted; (3) signals within one nucleus should have approximately the same size and intensity (thus excluding non-specific signals such as minor binding sites); and (4) parallel-cut sections stained with hematoxylin and eosin were evaluated simultaneously to identify cell types and tissue areas [21]. The number of FISH signals were counted on at least 100 nuclei per hybridized paraffin section. The cutoff levels for each probe were the mean percentage of cells with false positive signals plus 3 standard deviations, which were determined by analyzing 3 reactive tonsil tissues [23]. Cases showing poor FISH signals were reexamined, or examined using other available samples from the same patient.
Objective lymphoma response to therapy was assessed in all patients by biomicroscopic examination or orbital imaging study (computed tomography or magnetic resonance imaging) by experienced oculoplasty specialists. Objective response was defined according to the World Health Organization criteria. Complete remission was defined as the disappearance of all clinical evidence of the disease. Partial response was defined as more than 50% reduction in size of all measurable lesions. Stable disease was defined as regression of any measurable lesion by ≤50% (minimal response) or no change for the measurable lesions. Progressive disease was defined as the appearance of any new lesion or an increase in the size of a tumor of ≥25% at a previously involved site. Treatment failure was defined as conversion of treatment modality due to minimal response, local or systemic progression of lymphoma, or relapse (lymphoma recurrence after initial response).
Correlations between clinicopathologic parameters and genetic rearrangements were assessed by Fisher's exact test after transformation into categorical values. Overall survival was measured from the date of diagnosis to the date of death or last follow-up visit. Two-sided p-values <0.05 were considered to be statistically significant. Statistical analysis was performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA).
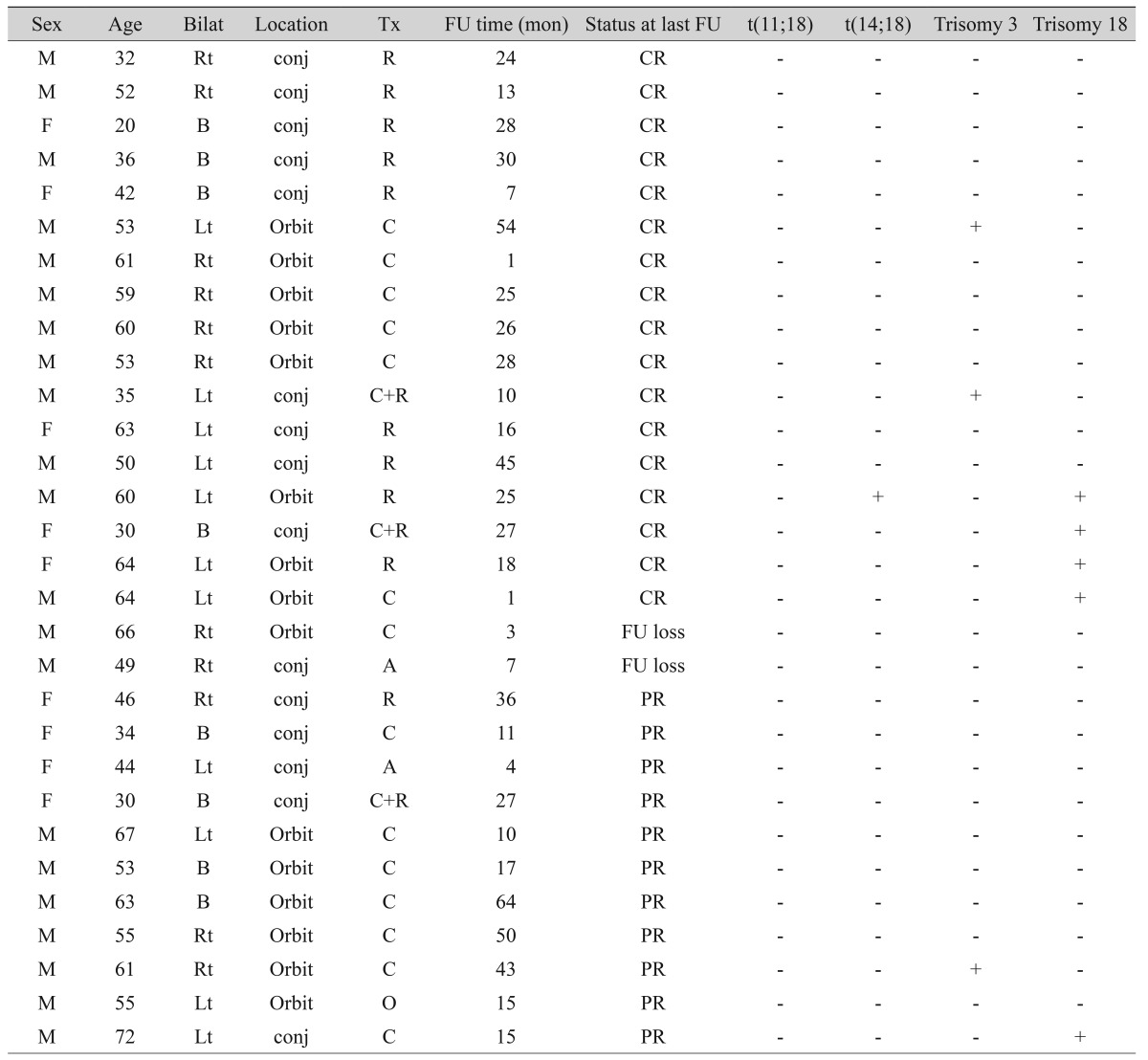
The clinical characteristics and FISH results of all 30 ocular adnexal MALT lymphoma patients are listed in Table 1. The mean patient age was 51.0 years (range, 20 to 72 years), and women were more affected than men (M : F = 9 : 21). Of the 30 cases, 15 (50%) were conjunctival MALT lymphomas and 15 (50%) orbital MALT lymphomas. Bilaterality was observed in 8 patients (26.7%).
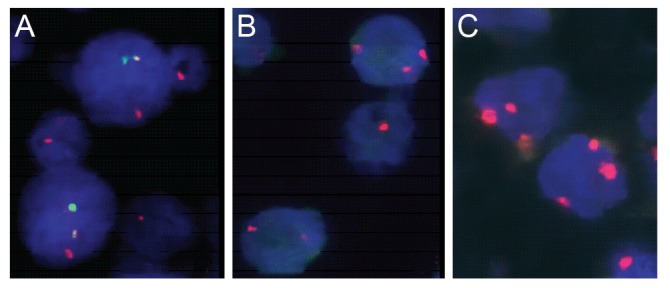
Using FISH analysis with break-apart probes, MALT1 rearrangement was noted in one out of 30 cases (3.4%). In a second step analysis to demonstrate the partner gene of this translocation, the t(14;18) IgH-MALT1 translocation was found in this case (Fig. 1A). The t(11;18) API2-MALT1 translocation was not observed.
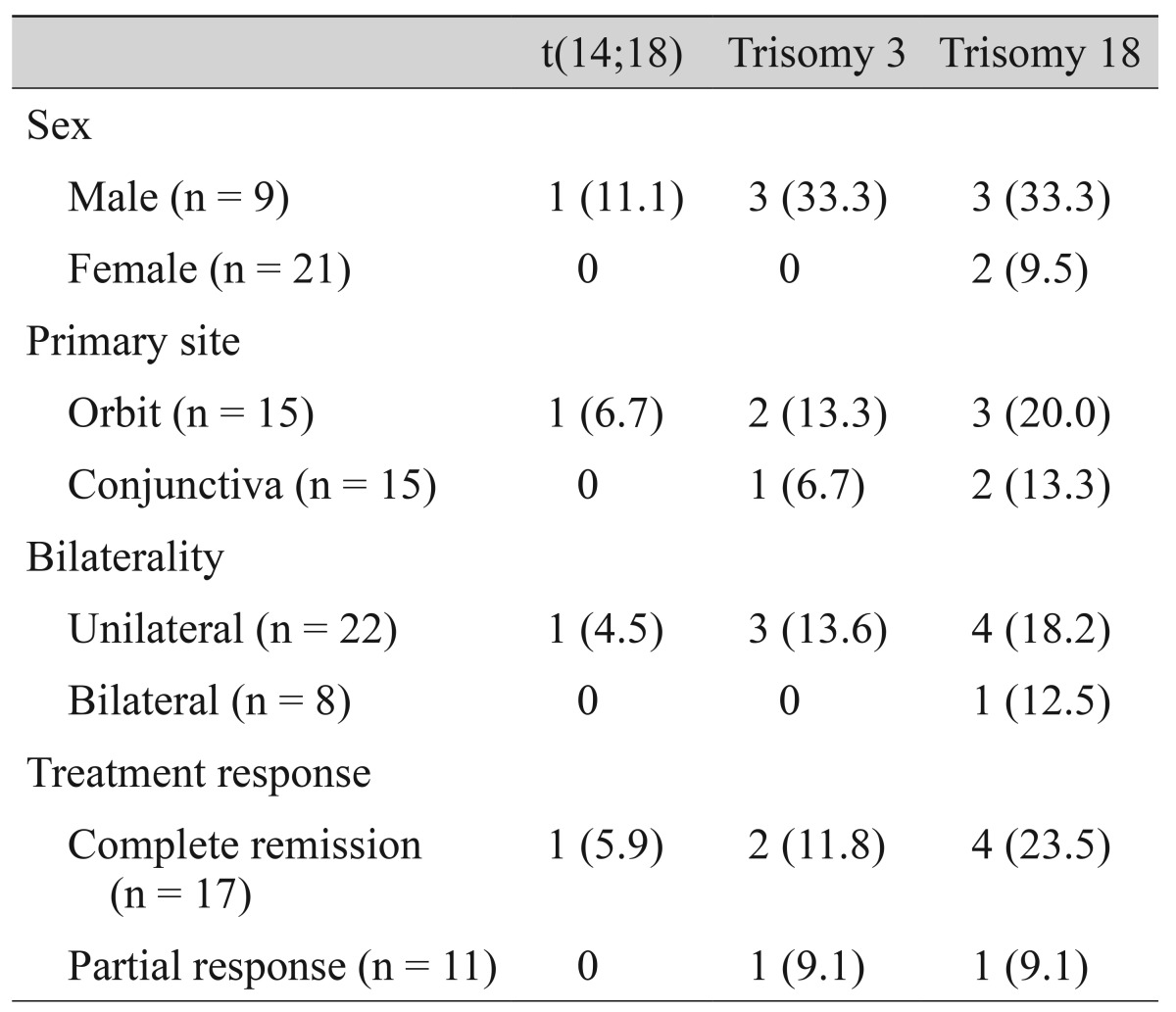
Numerical aberrations involving chromosome 3 or 18 were all trisomies. Trisomy 3 was observed in three ocular adnexal MALT lymphomas (10.0%) (Fig. 1B), and five cases showed trisomy 18 (16.7%) (Fig. 1C). Trisomy 3 and trisomy 18 were mutually exclusive. Overall, 8 of 30 cases (26.7%) showed trisomy 3 or 18. The one translocation positive case also showed trisomy 18. One recurrent case showed trisomy 18 only in recurrent biopsies.
There were no statistically significant differences in clinicopathologic parameters between trisomy or translocation-positive and negative groups (Table 2).
The follow-up period ranged from 1 to 64 months (22.7 ± 16.2, median 21.0 months). All ocular adnexal MALT lymphoma patients survived with 100% overall survival rate. Seventeen cases (60.7%) showed complete remission at the last follow-up visit. Two cases had bone marrow involvement at the time of initial diagnosis, and both cases showed no chromosomal aberrations. One of these cases recurred after chemotherapy and showed trisomy 18 only in recurrent biopsies. There were no statistically significant differences in prognosis between trisomy or translocation-positive and negative groups (Table 2).
Ocular adnexal lymphoma represents a significant proportion (approximately 12%) of all MALT lymphomas [20], and MALT lymphoma is the most common lymphoma of the ocular adnexa [24], occurring principally in the conjunctiva, orbital soft tissue, and lacrimal gland. The increasing incidence of ophthalmic lymphoma-and especially of ocular adnexal MALT lymphoma-calls for studies clarifying its pathogenesis. Indeed, the pathogenesis of MALT lymphoma is largely unknown, and there are currently no generally accepted prognostic factors for primary ocular adnexal MALT lymphoma.
The putative etiologic agent of ocular adnexal MALT lymphoma is thought to be Chlamydia psittaci (Cp) in the same way that H. pylori is thought to cause gastric MALT lymphoma [25,26]. The high proportion of gastric MALT lymphoma is likely due to the high prevalence of H. pylori in Korea, which is estimated to infect 75% of all adults [27]. Likewise, the infection rate for Cp has been reported to be very high in Korea, positing one explanation for the high prevalence of ocular adnexal MALT lymphoma in this population [8,28]. However, in contrast to the remark-able success of H. pylori eradication in gastric MALT lymphoma, ocular adnexal MALT lymphoma has shown inconsistent association with the purported pathogens and variable response to antibiotic treatment [29,30]. Therefore, ocular adnexal MALT lymphoma may have other etiologic factors that underlie its pathogenesis, such as genetic or epigenetic alterations, in addition to infection by Cp, that make the Korean population uniquely vulnerable [8,31,32,33,34]. In a study of non-Hodgkin's lymphoma in Asian Americans using SEER data from 1988 to 2004, Clarke et al. [35] report that the incidence of follicular lymphoma, CLL/SLL, and nodular sclerosis Hodgkin lymphoma are significantly higher in US-born Asians (second-generation immigrants or beyond) than in foreign-born Asians (first-generation immigrants), which supports the role of environmental factors in lymphomagenesis. A similar trend was observed in studies of resident Koreans [36,37]. Therefore the influence of cancer-causing behavior of a particular ethnic group within a certain environment cannot be excluded.
Recently, various genetic changes specific to MALT lymphoma, including numerical abnormalities of chromosomes 3, 7, 12, 18, and chromosomal translocations t(1;14) (p22;q14), t(11;18)(q21;q21), have been reported [15,16]. These genetic changes has also harbor clinical significance; for example, t(11;18)-positive cases are resistant to H. pylori eradication therapy [38,39]. The reported incidence of these chromosomal abnormalities have been inconsistent, which seems to be related to variety of the initial anatomic site of involvement [9,10].
In this study, we analyzed the clinical and cytogenetic characteristics of 30 Korean ocular MALT lymphoma patients. In contrast to previous reports, we found that ocular adnexal MALT lymphomas in this population are not located solely in the orbit, but rather occur with an even distribution in the conjunctiva and orbit [17,40]. We also found that the ocular adnexal MALT lymphomas in this Korean population were rarely disseminated, in contrast to those in other reports which showed more disseminated disease at presentation, more frequent relapses at extraocular sites, and lower overall survival rates with a 5-year overall survival of 75% and 10% of patients dying from their lymphoma [17]. Based on these clinical differences, we hypothesized that the frequencies of genetic aberrations also may differ according to the geographic location or ethnic groups.
We found that the t(14;18) IgH-MALT1 translocation was rare (3.3%), concordant with others reports showing frequencies ranging from 0% to 10% [9,17,41,42,43,44,45]. In contrast, the t(11;18)(q21;q21) API2-MALT translocation was not detected in any cases in our study, although this translocation has been reported in up to 13% in two studies conducted in Europe and Japan [20,22]. This finding is in line with the lower frequencies of this translocation in gastric MALT lymphomas of East Asians; although previous studies reported that approximately 30% of MALT lymphomas in western countries harbor the t(11;18) translocation [46,47], the incidence of this translocation in gastric MALT lymphomas is only 7.5% to 12.5% in East Asians [48,49]. Thus, the difference in frequencies of these aberrations suggest that not only site, but also geographical and environmental conditions, may play an important role in the type of genetic abnormalities in ocular adnexal MALT lymphoma.
One translocation positive case in this study was accompanied by trisomy 18, which is not common in gastric MALT lymphoma with t(11;18) API2-MALT 1 translocation [49,50]. In fact, numeric aberrations and the API2-MALT1 translocation have been reported to be mutually exclusive. This conflicting result might be explained by the different anatomical sites of origin or by different translocation partner genes. Future studies with more cases will be needed to clarify this result.
Interestingly, one case of patient relapse in this study showed trisomy 18 only in the relapsed specimen. There have been some reports that chromosomal aneuploidy such as trisomy 3, 18 are associated with high grade transformation of low grade MALT lymphoma [49,50]. Though a microscopically high grade component was not observed in this case, the trisomy 18 we detected might ref lect the treatment-resistant nature of the tumor.
In conclusion, chromosomal translocations involving the MALT1 gene are not a common finding in Korean ocular adnexal MALT lymphomas. The t(14;18) translocation was detected in only one case, and the t(11;18) translocation was not found at all. Therefore, chromosomal aberrations may have no prognostic implications in this patient population.
Acknowledgements
This work was supported by grant no 04-2005-0270 from the Seoul National University Hospital Research Fund.
Conflicts of interest
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Cho EY, Han JJ, Ree HJ, et al. Clinicopathologic analysis of ocular adnexal lymphomas: extranodal marginal zone b-cell lymphoma constitutes the vast majority of ocular lymphomas among Koreans and affects younger patients. Am J Hematol 2003;73:87-96.


2. Coupland SE, Hummel M, Stein H. Ocular adnexal lymphomas: five case presentations and a review of the literature. Surv Ophthalmol 2002;47:470-490.


3. Takino H, Li C, Hu S, et al. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathological study of cases from Asia, Germany, and the United States. Mod Pathol 2008;21:1517-1526.


4. Moslehi R, Devesa SS, Schairer C, Fraumeni JF Jr. Rapidly increasing incidence of ocular non-hodgkin lymphoma. J Natl Cancer Inst 2006;98:936-939.


5. Oh DE, Kim YD. Lymphoproliferative diseases of the ocular adnexa in Korea. Arch Ophthalmol 2007;125:1668-1673.


6. Huang Q, Su X, Ai L, et al. Promoter hypermethylation of multiple genes in gastric lymphoma. Leuk Lymphoma 2007;48:1988-1996.


7. Liu XF, Kong FM, Xu Z, et al. Promoter hypermethylation of death-associated protein kinase gene in cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2007;6:407-411.

8. Choung HK, Kim YA, Lee MJ, et al. Multigene methylation analysis of ocular adnexal MALT lymphoma and their relationship to Chlamydophila psittaci infection and clinical characteristics in South Korea. Invest Ophthalmol Vis Sci 2012;53:1928-1935.


9. Remstein ED, Dogan A, Einerson RR, et al. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol 2006;30:1546-1553.


10. Streubel B, Simonitsch-Klupp I, Mullauer L, et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia 2004;18:1722-1726.


11. Du MQ, Atherton JC. Molecular subtyping of gastric MALT lymphomas: implications for prognosis and management. Gut 2006;55:886-893.



12. Liu H, Ye H, Ruskone-Fourmestraux A, et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology 2002;122:1286-1294.


13. Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001;98:1182-1187.


14. Inagaki H. Mucosa-associated lymphoid tissue lymphoma: molecular pathogenesis and clinicopathological significance. Pathol Int 2007;57:474-484.


15. Wotherspoon AC, Pan LX, Diss TC, Isaacson PG. Cytogenetic study of B-cell lymphoma of mucosa-associated lymphoid tissue. Cancer Genet Cytogenet 1992;58:35-38.


16. Akagi T, Motegi M, Tamura A, et al. A novel gene, MALT1 at 18q21, is involved in t(11;18) (q21;q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Oncogene 1999;18:5785-5794.


17. Sjo LD, Heegaard S, Prause JU, et al. Extranodal marginal zone lymphoma in the ocular region: clinical, immunophenotypical, and cytogenetical characteristics. Invest Ophthalmol Vis Sci 2009;50:516-522.


18. Takada S, Yoshino T, Taniwaki M, et al. Involvement of the chromosomal translocation t(11;18) in some mucosa-associated lymphoid tissue lymphomas and diffuse large B-cell lymphomas of the ocular adnexa: evidence from multiplex reverse transcriptase-polymerase chain reaction and fluorescence in situ hybridization on using formalin-fixed, paraffin-embedded specimens. Mod Pathol 2003;16:445-452.


19. Ye H, Gong L, Liu H, et al. MALT lymphoma with t(14;18) (q32;q21)/IGH-MALT1 is characterized by strong cytoplasmic MALT1 and BCL10 expression. J Pathol 2005;205:293-301.


20. Isaacson PG, Chott A, Nakamura S, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Swerdlow S, Campo E, Harris NL, WHO classification of tumors of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. p. 214-217.
21. Ye H, Liu H, Attygalle A, et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood 2003;102:1012-1018.


22. Hoeve MA, Gisbertz IA, Schouten HC, et al. Gastric low-grade MALT lymphoma, high-grade MALT lymphoma and diffuse large B cell lymphoma show different frequencies of trisomy. Leukemia 1999;13:799-807.


23. Murga Penas EM, Hinz K, Roser K, et al. Translocations t(11;18)(q21;q21) and t(14;18)(q32;q21) are the main chromosomal abnormalities involving MLT/MALT1 in MALT lymphomas. Leukemia 2003;17:2225-2229.


24. Jenkins C, Rose GE, Bunce C, et al. Histological features of ocular adnexal lymphoma (REAL classification) and their association with patient morbidity and survival. Br J Ophthalmol 2000;84:907-913.



25. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991;338:1175-1176.


26. Ferreri AJ, Guidoboni M, Ponzoni M, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004;96:586-594.


27. Malaty HM, Kim JG, Kim SD, Graham DY. Prevalence of Helicobacter pylori infection in Korean children: inverse relation to socioeconomic status despite a uniformly high prevalence in adults. Am J Epidemiol 1996;143:257-262.


28. Yoo C, Ryu MH, Huh J, et al. Chlamydia psittaci infection and clinicopathologic analysis of ocular adnexal lymphomas in Korea. Am J Hematol 2007;82:821-823.


29. Kim TM, Kim KH, Lee MJ, et al. First-line therapy with doxycycline in ocular adnexal mucosa-associated lymphoid tissue lymphoma: a retrospective analysis of clinical predictors. Cancer Sci 2010;101:1199-1203.


30. Han JJ, Kim TM, Jeon YK, et al. Long-term outcomes of first-line treatment with doxycycline in patients with previously untreated ocular adnexal marginal zone B cell lymphoma. Ann Hematol 2015;94:575-581.


31. Daibata M, Nemoto Y, Togitani K, et al. Absence of Chlamydia psittaci in ocular adnexal lymphoma from Japanese patients. Br J Haematol 2006;132:651-652.


32. Rosado MF, Byrne GE Jr, Ding F, et al. Ocular adnexal lymphoma: a clinicopathologic study of a large cohort of patients with no evidence for an association with Chlamydia psittaci. Blood 2006;107:467-472.



33. Mulder MM, Heddema ER, Pannekoek Y, et al. No evidence for an association of ocular adnexal lymphoma with Chlamydia psittaci in a cohort of patients from the Netherlands. Leuk Res 2006;30:1305-1307.


34. Vargas RL, Fallone E, Felgar RE, et al. Is there an association between ocular adnexal lymphoma and infection with Chlamydia psittaci? The University of Rochester experience. Leuk Res 2006;30:547-551.


35. Clarke CA, Glaser SL, Gomez SL, et al. Lymphoid malignancies in U.S. Asians: incidence rate differences by birthplace and acculturation. Cancer Epidemiol Biomarkers Prev 2011;20:1064-1077.



36. Yoon SO, Suh C, Lee DH, et al. Distribution of lymphoid neoplasms in the Republic of Korea: analysis of 5318 cases according to the World Health Organization classification. Am J Hematol 2010;85:760-764.


37. Kim JM, Ko YH, Lee SS, et al. WHO classification of malignant lymphomas in Korea: report of the third nationwide study. Korean J Pathol 2011;45:254-260.

38. Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001;357:39-40.


39. Alpen B, Neubauer A, Dierlamm J, et al. Translocation t(11;18) absent in early gastric marginal zone B-cell lymphoma of MALT type responding to eradication of Helicobacter pylori infection. Blood 2000;95:4014-4015.


40. Ferry JA, Fung CY, Zukerberg L, et al. Lymphoma of the ocular adnexa: a study of 353 cases. Am J Surg Pathol 2007;31:170-184.


41. Sagaert X, Laurent M, Baens M, et al. MALT1 and BCL10 aberrations in MALT lymphomas and their effect on the expression of BCL10 in the tumour cells. Mod Pathol 2006;19:225-232.


42. Adachi A, Tamaru J, Kaneko K, et al. No evidence of a correlation between BCL10 expression and API2-MALT1 gene rearrangement in ocular adnexal MALT lymphoma. Pathol Int 2004;54:16-25.


43. Ruiz A, Reischl U, Swerdlow SH, et al. Extranodal marginal zone B-cell lymphomas of the ocular adnexa: multiparameter analysis of 34 cases including interphase molecular cytogenetics and PCR for Chlamydia psittaci. Am J Surg Pathol 2007;31:792-802.


44. Schiby G, Polak-Charcon S, Mardoukh C, et al. Orbital marginal zone lymphomas: an immunohistochemical, polymerase chain reaction, and fluorescence in situ hybridization study. Hum Pathol 2007;38:435-442.


45. Tanimoto K, Sekiguchi N, Yokota Y, et al. Fluorescence in situ hybridization (FISH) analysis of primary ocular adnexal MALT lymphoma. BMC Cancer 2006;6:249



46. Ott G, Katzenberger T, Greiner A, et al. The t(11;18) (q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin's lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res 1997;57:3944-3948.

47. Dierlamm J, Baens M, Stefanova-Ouzounova M, et al. Detection of t(11;18)(q21;q21) by interphase fluorescence in situ hybridization using API2 and MLT specific probes. Blood 2000;96:2215-2218.


48. Nomura K, Yoshino T, Nakamura S, et al. Detection of t(11;18)(q21;q21) in marginal zone lymphoma of mucosa-associated lymphocytic tissue type on paraffin-embedded tissue sections by using fluorescence in situ hybridization. Cancer Genet Cytogenet 2003;140:49-54.


Fig. 1
Fluorescence in situ hybridization detection of IgH-MALT1 translocation, trisomy 3 and 18 in ocular adnexal mucosa associated lymphoid tissue (MALT) lymphomas. (A) Green signal represents IgH and red signal MALT1 gene. Yellow fusion signals (red+green) were detected in a t(14;18)-positive case of ocular MALT lymphoma using dual color dual fusion translocation probe. (B,C) Red signals represent centromeric regions of chromosome 3 (B) or 18 (C). Three red signals were shown in trisomy 3 or 18 of ocular adnexal MALT lymphomas, respectively.
- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




