|
 |
| Korean J Ophthalmol > Volume 28(1); 2014 > Article |
Abstract
We report a case of complete surgical resolution of Valsalva retinopathy that manifested as a premacular hemorrhage involving a membrane followed by a macular hole (MH) resulting from the first vitrectomy. A 20-year-old female patient was referred to our hospital due to sudden vision loss in the left eye. Her best-corrected visual acuity (BCVA) in the left eye was hand motion. Fundus photographs and optical coherence tomography (OCT) revealed a premacular hemorrhage. Nine weeks later, the BCVA in the left eye had returned to 20 / 100 and the premacular hemorrhage had completely resolved, but residual sub-internal limiting membrane deposits and a preretinal membrane were present. The preretinal membrane was removed by core vitrectomy and preretinal membrane peeling, but the foveal deposits could not be excised. Two weeks after the first vitrectomy, the deposits resolved spontaneously, but a full-thickness MH was present. Six months after a second vitrectomy with fluid-gas exchange, the BCVA in the left eye had improved to 20 / 25 and OCT showed that the MH had closed. This case illustrates the possibility of MH formation following vitrectomy for Valsalva retinopathy.
The Valsalva maneuver involves forcible exhalation against the closed glottis and produces a sudden increase in the venous blood pressure due to an increase in intrathoracic or intraabdominal pressure. Valsalva hemorrhagic retinopathy was first described by Duane [1] in 1973 as preretinal hemorrhages observed in association with heavy lifting, vomiting, straining, or coughing. This type of retinopathy is caused by rupture of the superficial retinal capillaries due to increased intraocular venous pressure secondary to an abrupt increase in intrathoracic or intraabdominal pressure and results in sudden, painless loss of vision in an otherwise healthy eye. The hemorrhage typically occurs at the macula and, in the vast majority of cases, resolves spontaneously without compromising visual acuity. Generally, it is an isolated and self-limited event, but even a small premacular hemorrhage of one disc diameter (DD) may take several months to clear [2].
To the best of our knowledge, this is the first reported case of complete surgical resolution of Valsalva retinopathy that manifested as a premacular hemorrhage with membrane followed by macular hole (MH) formation resulting from the first vitrectomy. We describe changes observed with spectral domain-optical coherence tomography (SD-OCT) following two separate 23-gauge pars plana vitrectomy procedures to treat the MH formed as a complication of Valsalva retinopathy.
A 20-year-old female patient was referred to our hospital due to a sudden decrease in visual acuity in the left eye after heavy alcohol consumption. She denied any history of trauma or sexual contact. Her medical and ocular histories were unremarkable. Previous medical records reported that the patient had a history of severe vomiting following heavy drinking. In the left eye, the best-corrected visual acuity (BCVA) was hand motion. The anterior segment was unremarkable in both eyes. Fundus examination was normal in the right eye but revealed a well-circumscribed premacular hemorrhage about 4 DDs in size beneath a transparent membrane with glistening reflexes extending over the macula in the left eye. No posterior vitreous detachment (PVD) was evident. Time-domain OCT (TD-OCT) revealed an intact foveal contour and two membranes of differing optical reflectivity, identified as the internal limiting membrane (ILM) and the posterior hyaloid, respectively, with blood beneath the hyperreflective membrane (Fig. 1).
During a nine-week follow-up period, the premacular hemorrhage resolved spontaneously, and the BCVA in the left eye returned to 20 / 100. Yellowish residual sub-ILM deposits, however, were present, and a thick preretinal membrane was visible over the fovea (Fig. 1).
The patient requested aggressive treatment. After full explanation of the expected effects and possible complications of vitrectomy, the patient provided informed consent. A 23-gauge pars plana vitrectomy was performed using a minimal core vitrectomy technique. PVD was created using triamcinolone acetonide and a vitreous cutter only around the posterior pole. The ILM was peeled off using 23-gauge microforceps after staining the ILM with indocyanine green (Diagnogreen; Daiichi Pharmaceutical, Tokyo, Japan). The ILM was removed from a 2- to 3-DD area centered on the fovea. The thick preretinal membrane and the ILM were removed, but the residual foveal deposits could not be extracted because they had adhered to the fovea. The surgeon expected the deposits to absorb spontaneously, so fluid-air exchange was not performed. Two weeks after the first vitrectomy, the foveal deposits had resolved, but a full thickness MH was observed. Postoperative SD-OCT, which was used to analyze the cross-sectional image almost every day (Fig. 2), did not reveal an MH during the first seven days. A second vitrectomy was performed using standard techniques to create a total PVD and to remove the remnant peripheral vitreous with fluid-air exchange through an extrusion cannula. The eye was flushed with a 15% perfluoropropane gas-air mixture to ensure complete exchange. After the second surgery, the patient was instructed to maintain a strict face-down position for three days in the hospital until MH closure was confirmed.
Six months after the second surgery to close the MH, the BCVA in the left eye had improved to 20 / 25. SD-OCT showed that the MH had closed with only slight scarring of the photoreceptor inner segment/outer segment junction (Fig. 3).
Valsalva retinopathy develops in response to the Valsalva maneuver. Reported causes of Valsalva retinopathy include straining and physical activities, most commonly coughing, weight lifting, vomiting, aerobic exercise, sexual activity, colonoscopy procedures, and congenital retinal macroaneurysm [3-6]. A history of such activities is helpful in establishing this diagnosis. Although in the present case, the patient did not remember the exact situation due to heavy drinking, according to the previous medical records, severe vomiting could have been a possible cause of Valsalva retinopathy.
The presentation of Valsalva retinopathy varies depending on the size of the vessel involved and the location of the hemorrhage, which can be subretinal, intraretinal and/or subhyaloid. The exact location of premacular hemorrhage (whether sub-hyaloid or sub-ILM) has been disputed in the literature. Hemorrhage following Valsalva retinopathy is more commonly sub-ILM than sub-hyaloid; rarely, it may be a combination of both [2]. In the present case, premacular hemorrhage manifested as a small dome-shaped, sub-ILM hemorrhage that was characterized by a glistening light reflex, fine striae on the surface of the hemorrhage, and a large dome-shaped sub-hyaloid hemorrhage located anterior and inferior to the sub-ILM hemorrhage, as determined by color fundus photographs and SD-OCT (Fig. 1).
To the best of our knowledge, development of an MH after vitrectomy to remove the thick preretinal membrane following spontaneous resolution of the premacular hemorrhage has not been previously reported in the literature. There are several possible mechanisms that could explain MH formation after the initial vitrectomy.
First, the MH could be iatrogenic (caused by ILM peeling). Before the first procedure, a thick preretinal membrane and sub-ILM deposits over the foveal area were observed by SD-OCT. If the MH had been iatrogenic, we would have been able to observe it immediately after surgery using SD-OCT. However, post-surgical SD-OCT images did not reveal a MH, which could be due to the sticky nature of foveal deposits (Fig. 2).
Second, the thick preretinal membrane observed after resolution of the premacular hemorrhage was considered to create a tangential tractional force over the fovea [7]. Due to this tangential force, the increased volume of the sub-ILM deposits under the thick preretinal membrane was likely to provide an additional outward expanding force into the fovea. It is possible that the thick preretinal membrane prevented formation of the MH. After the residual foveal deposit was absorbed, the MH occurred with a fading holding effect of a sticky deposit, which sustained the margin of the MH.
The prognosis of Valsalva retinopathy is generally good, and the condition in most patients resolves spontaneously over several months. In the majority of cases, conservative management is indicated with periodic observation. Therapeutic options in Valsalva retinopathy include conservative management, vitrectomy [8], laser membranotomy [9], intravitreal tissue plasminogen activator, and tamponade gas injection [10]. If sub-ILM and sub-hyaloid hemorrhage were present in the macular area in the presented case, we would have considered yttrium-aluminum-garnet(YAG) laser and tissue plasminogen activator as a treatment option. The sub-ILM and sub-hyaloid hemorrhages, however, were absorbed completely, and only the yellowish sub-ILM deposits remained. Accordingly, we did not choose YAG laser and tissue plasminogen activator as a treatment option. In the present case, the MH seemed to be a possible complication after vitrectomy to remove the thick preretinal membrane associated with Valsalva retinopathy.
The residual sub-ILM deposits in the fovea were not absorbed spontaneously before the first vitrectomy; therefore, the patient's visual acuity did not improve. After ILM peeling following the first vitrectomy, the surgeon waited for spontaneous absorption of the deposits. Two weeks after the first procedure, the residual sub-ILM deposits had completely resolved, and the MH could be clearly diagnosed. A second operation was then performed with standard techniques to close the MH. The ILM peeling that occurred during the first surgery, however, likely accelerated the spontaneous absorption of the residual foveal deposits and might have contributed to the closing of the MH. Delaying the first operation may have led to a prolonged time for absorption of the deposits, thus worsening the prognosis due to the presence of composites such as iron in the deposits or organizing scar tissue, and the MH would have continued to be masked by the deposits.
In conclusion, patients should be monitored carefully for the development of a MH after vitrectomy for Valsalva retinopathy with residual sub-ILM deposits, as described in the present case.
Notes
This paper was presented as a "Challenging Case of Symposia" at the 27th Asia-Pacific Academy of Ophthalmology meeting in April 2012.
REFERENCES
2. Gass JD. Stereoscopic atlas of macular diseases: diagnosis and treatment. 4th ed. St. Louis: Mosby; 1997. p. 752-754.
3. Roberts DK, MacKay KA. Microhemorrhagic maculopathy associated with aerobic exercise. J Am Optom Assoc 1987;58:415-418.

4. Friberg TR, Braunstein RA, Bressler NM. Sudden visual loss associated with sexual activity. Arch Ophthalmol 1995;113:738-742.


5. De Crecchio G, Pacente L, Alfieri MC, Greco GM. Valsalva retinopathy associated with a congenital retinal macrovessel. Arch Ophthalmol 2000;118:146-147.


6. Choi SW, Lee SJ, Rah SH. Valsalva retinopathy associated with fiberoptic gastroenteroscopy. Can J Ophthalmol 2006;41:491-493.


7. Kwok AK, Lai TY, Chan NR. Epiretinal membrane formation with internal limiting membrane wrinkling after Nd:YAG laser membranotomy in valsalva retinopathy. Am J Ophthalmol 2003;136:763-766.


8. De Maeyer K, Van Ginderdeuren R, Postelmans L, et al. Sub-inner limiting membrane haemorrhage: causes and treatment with vitrectomy. Br J Ophthalmol 2007;91:869-872.



Fig.┬Ā1
(A) A fundus photograph of the left eye shows a dome-shaped sub-internal limiting membrane (ILM) hemorrhage (white asterisk) and a sub-hyaloid hemorrhage located anterior and inferotemporal to the sub-ILM hemorrhage (white cross) at initial presentation. Note the "double ring" sign with the "inner ring" caused by the sub-ILM hemorrhage and the "outer ring" caused by the sub-hyaloid hemorrhage. (B) After four weeks, a fundus photograph showed spontaneous absorption of the sub-hyaloid hemorrhage, but the sub-ILM hemorrhage remained. (C) After nine weeks, the sub-ILM and sub-hyaloid hemorrhages were almost completely resolved, and a focal yellowish lesion was observed on the fovea. (D) The Time-domain optical coherence tomography (OCT) at the initial presentation revealed a premacular hemorrhage with hyperreflective ILM (white arrowhead) and a hyporeflective sub-hyaloid membrane (white arrow). The foveal contour was intact (double arrow). (E) After four weeks, OCT showed resolution of the premacular hemorrhage with a thick preretinal membrane. (F) After nine weeks, the premacular hemorrhage was completely resolved, but a hyperreflective, thick preretinal membrane (white arrowhead) remained.
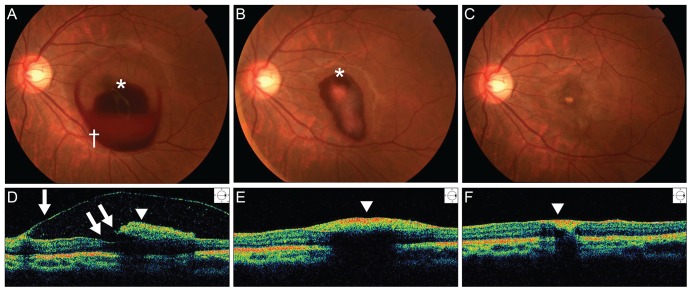
Fig.┬Ā2
Serial spectral domain-optical coherence tomography images before and after pars plana vitrectomy. The macular hole was not identified immediately after the first operation but was observed after resolution of the sub-internal limiting membrane deposits at two weeks after the first surgery. (A) Before 1st operation, (B) 1 day after 1st operation, (C) 4 day after 1st operation, (D) 7 day after 1st operation, (E) 14 day after 1st operation, and (F) 14 day after 2nd operation.
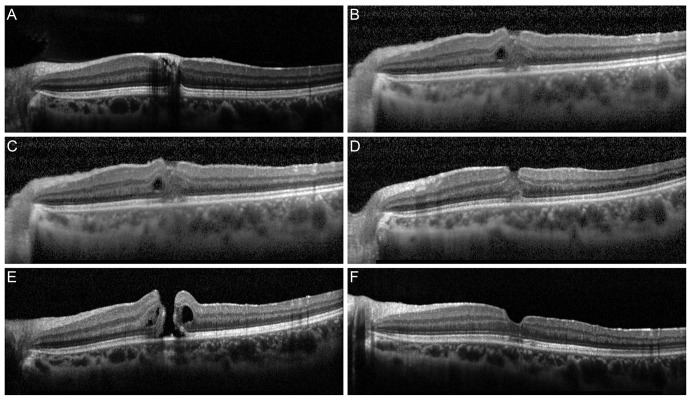
Fig.┬Ā3
(A,B) Fundus photograph and spectral domain-optical coherence tomography (SD-OCT) images after the initial pars plana vitrectomy. SD-OCT revealed hemosiderin-like deposits (arrowhead) of the fovea. (C,D) Fundus photograph and SD-OCT images before secondary pars plana vitrectomy. SD-OCT showed a full-thickness macular hole. (E,F) Six months after the second surgery, the macular hole was completely closed with minimal scarring of the photoreceptor inner/outer segment junction (arrow), as determined by SD-OCT.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print