 |
 |
| Korean J Ophthalmol > Volume 28(6); 2014 > Article |
Abstract
Purpose
To evaluate plasma pentraxin 3 (PTX3) in patients with retinal vein occlusion (RVO), and investigate the possibility of its role as a predictive biomarker.
Methods
Nested case-control study. The study included 57 patients with RVO and 45 age- and gender-matched subjects without RVO as controls. Plasma PTX3 and C-reactive protein concentration were measured in both groups a posteriori from frozen samples by using an enzyme-linked immunosorbent assay kit.
Results
The measured PTX3 value for the RVO group was 1,508 ± 1,183 pg/mL (mean ± standard deviation) and 833 ± 422 pg/mL for the controls (p < 0.001). There was no significant difference in PTX3 levels between patients with central retinal vein occlusion and branched retinal vein occlusion (1,468 ± 1,300 vs. 1,533 ± 1,121 pg/mL; p = 0.818).
Retinal vein occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy. In most cases, RVO is caused by a reduction in venous flow, which manifests itself either as a central retinal vein occlusion (CRVO) or branch retinal vein occlusion (BRVO) [1].
Associated macular edema can appear after multifactorial pathophysiologic changes resulting in compromised capillary flow and breakdown of the blood-retinal barrier with a subsequent imbalance of angiogenic and inflammatory cytokines in the ocular fluid [2]. Cytokines therefore play a crucial role in the functioning of endothelial cells and leucocytes, which contribute to cytokine secretion [3]. The long-term exposure of endothelial cells to proinflammatory cytokines can lead to leucocyte extravasation and thrombosis, in addition to the originating retinal venous obstruction. Thus, inflammation and vascular dysfunction interact with each other and are stimulated predominantly by vascular endothelial growth factor, a known chemoattractant cytokine for macrophages and leucocytes. These findings explain the upregulation of vascular endothelial growth factor mRNA in RVO [4].
Some studies have reported that RVO is associated with increased levels of acute-phase proteins such as the highly sensitive C-reactive protein (CRP), which is related to the innate immune response and inflammation [5]. Other studies identified highly sensitive CRP as a predictor of large vessel disease in healthy subjects [6], and showed its association with the development of peripheral vascular disease [7].
Pentraxins are a superfamily of acute phase reactants characterized by a cyclic multimeric structure. The classical short pentraxin, CRP, belongs to the pentraxin protein family which is structurally distinguished by a characteristic pentameric structure [8]. The long pentraxin family member, pentraxin 3 (PTX3), was recently described. Like CRP, PTX3 is induced by acute inflammatory stimuli such as bacterial products, interleukin (IL)-1, and tumor necrosis factor (TNF). These stimuli are mediated by diverse cell types, predominantly macrophages and vascular endothelial cells, but, importantly, not by hepatocytes [9]. PTX3 is produced in vascular endothelial cells and macrophages, while CRP is produced mainly in hepatocytes predominantly under transcriptional control by the cytokine IL-6 [9,10]. Because of these facts, PTX3 levels may more directly reflect the inflammatory status of the vasculature. A recent study reported an elevation of inflammatory factors including PTX3 in the vitreous f luid of patients with CRVO [11]. However, to the best of our knowledge, no study has reported plasma PTX3 levels in patients with CRVO and BRVO.
This study evaluated plasma levels of PTX3 in patients with RVO and investigated the possibility of their role as a diagnostic biomarker. Accordingly, this study will provide insight into the etiological mechanisms involved in RVO.
This study was a nested case-control study. Fifty-seven RVO patients and 40 control subjects were enrolled from the Department of Ophthalmology, Ulsan University Hospital, Ulsan University College of Medicine between March 2010 and December 2012. This study was approved by the institutional human experimentation committee review board of Ulsan university hospital and was performed in accordance with the ethical standards in the 1964 Declaration of Helsinki. After a detailed explanation of the procedure benefits and risks, informed consent was obtained from all patients.
Both case and control subjects went through a complete ophthalmic examination. All fundus exams were performed and graded by a single retinal specialist. RVO was diagnosed when a retinal nonperfused area in an occluded area was detected by fluorescein angiography (FAG) with the Heidelberg Retina Angiograph Spectralis (Heidelberg Engineering, Heidelberg, Germany). FAG or fundus examination of these patients revealed retinal hemorrhages and dilated, tortuous retinal venous systems with or without macular edema. Forty age- and gender-matched healthy subjects without preexisting ocular disease were included as a control group for comparison.
Full eye examinations, including best-corrected visual acuity, tonometry, biomicroscopy, and fundoscopy after pupil dilation were performed in all subjects. Fundus photography and optical coherence tomography was done in all subjects to reveal macular edema, and FAG was done in all RVO patients and some controls when required.
Patients with RVO were classified as having CRVO or BRVO according to fundus exams and FAG. CRVO was diagnosed in the presence of four quadrants of retinal hemorrhage, vascular tortuosity, dilation, and delayed central retinal venous filling on fluorescein angiogram. If only one quadrant contained retinal hemorrhages, vascular tortuosity, dilation and delayed central retinal venous filling then BRVO was diagnosed.
Exclusion criteria included acute or chronic infectious diseases, autoimmune disorders or chronic inflammatory disease, creatinine more than 2.0 mg/dL, abnormal hepatic function tests (more than two-fold elevation), abnormal leukocyte count, and malignancies. Patients with ophthalmic problems known to be associated with inflammation or autoimmune disease and other ocular disease such as glaucoma, age-related macular degeneration, diabetic retinopathy, central serous chorioretinopathy and uveitis were also excluded.
Venous blood samples were obtained from patients before adding any antioxidant medication. The plasma was immediately separated by centrifugation and then stored at -80℃. Plasma PTX3 concentration was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) and plasma CRP concentration was measured with a highly sensitive ELISA kit (DakoCytomation, Glostrup, Denmark).
The statistical analyses were performed with SPSS ver. 18 (SPSS Inc., Chicago, IL, USA) using the independent t-test and the Mann-Whitney test. A p-value less than 0.05 was assumed to be statistically significant.
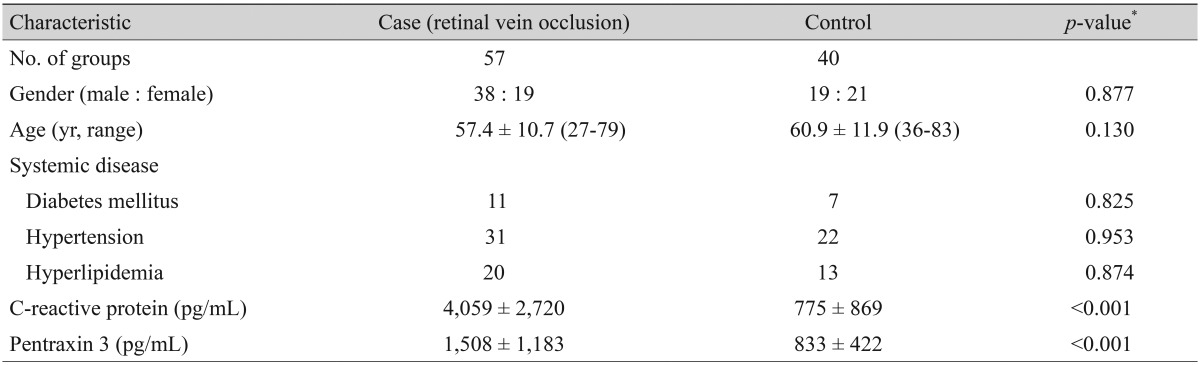
Baseline demographic characteristics are provided in Table 1. The case subjects included 38 men and 19 women ranging from 27 to 79 years of age (57.4 ± 10.7). Of the 40 controls, there were 19 men and 21 women ranging from 36 to 83 years of age (60.9 ± 11.9). There was no significant difference in the gender, age, and distribution of systemic diseases including diabetes and cardiovascular disease between patients with RVO and the control group.
Laboratory findings for each study group are summarized in Table 1. The CRP level was significantly higher among patients with RVO (4,059 ± 2,720 vs. 775 ± 869 pg/mL; p < 0.001). The measured PTX3 concentration for RVO patients was 1508 ± 1183 pg/mL, and was 833 ± 422 pg/mL for the controls (p < 0.001).
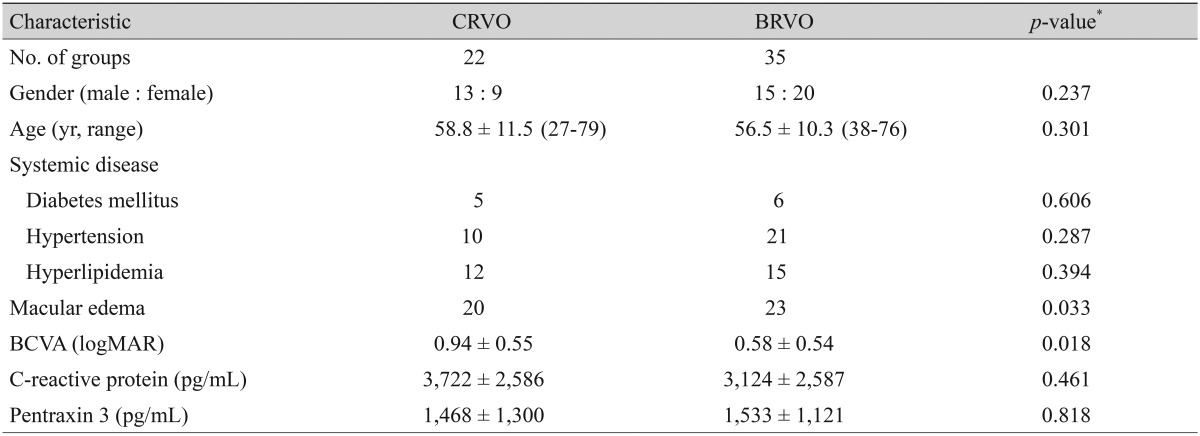
The baseline demographic characteristics and laboratory findings for the RVO subtype study groups are summarized in Table 2. The 22 CRVO subjects made up of 13 men and 9 women ranged from age 27 to 79 years (58.8 ± 11.5). Of the 35 BRVO subjects, there were 15 men and 20 women with ages ranging from 38 to 76 years (56.5 ± 10.3). The only baseline characteristics that appeared different between the subgroups was a best-corrected visual acuity (BCVA) that was significantly worse (0.94 ± 0.55 vs. 0.58 ± 0.54, p =0.018) and a relative prevalence of macular edema that was significantly higher (20 / 22 vs. 23 / 35, p = 0.033) in patients with CRVO. However, CRP and PTX3 concentrations were not significantly different between subjects with CRVO and BRVO (3,722 ± 2,586 vs. 3,124 ± 2,587 pg/mL, p = 0.461; 1,468 ± 1,300 vs. 1,533 ± 1,121 pg/mL, p = 0.818, respectively).
PTX3 is a recently described multimeric inflammatory mediator structurally linked to the short pentraxins like CRP [12]. While CRP is exclusively derived from hepatocytes, PTX3 can be synthesized by a variety of tissues and cells, such as vascular endothelial cells [13], macrophages [14], and fat tissue [15]. Moreover, our other study confirmed that human retinal pigment epithelial cells may be a major source of PTX3 production in the presence of proinflammatory cytokines such as IL-1β and TNF-α, and could be an important mediator for the inflammatory response in the retina [16]. Because PTX3 modulates the arrangement of leukocytes during the inflammatory process [17], an increment of PTX3 could lead to increased vascular permeability [18]. In fact, it is reported that PTX3-deficient mice have a more limited degree of vascular permeability in response to inflammatory signals [18]. Elevation of vascular permeability may be involved in the interaction among various inflammatory factors, so it is possible that increased vascular permeability due to increased PTX3 exacerbates macular edema associated with RVO. Thus, it is expected that PTX3 will act as a better biological marker of local tissue inflammation than conventional CRP.
Inflammation plays a key role in atherosclerosis [18,19,20]. Acute-phase reactants such as CRP and PTX3 are well known to be involved in the inflammatory response and atherosclerosis [9,21]. The increase of these protein in cardiovascular diseases implies that they can serve as a prognostic factor for vascular disease [22,23,24]. In addition, evidence suggests that PTX3 is released as part of a response specific to vascular damage, indicating that PTX3 may give information more pertinent to the development and progression of atherosclerosis than non-specific markers, such as CRP [25].
Recent reports detailed an increase of PTX3 after surgical procedures and myocardial infarctions with a response that was quicker, but less apparent than for CRP. One explanation is that PTX3 reflects baseline atherosclerotic ischemia more accurately in acute stress conditions such as stroke, myocardial infarctions, and RVO [26,27]. It may be productive for future studies to explore the specific functions and mechanisms of PTX3 in ischemic diseases.
The pathogenesis of RVO has been examined in numerous epidemiological, pathological, and biological studies and several mechanisms of pathogenesis have been proposed [28,29,30]. Martin et al. [28] concluded that a RVO can be the presenting sign in patients at increased risk for cardiovascular disease, and there may be increased mortality from cardiovascular disease in patients with vein occlusions. Since atherosclerotic plaques include proteins common to inflammation and immune-mediated processes, chronic inflammation and ischemia has been suggested to be one of the pathogenic mechanisms for RVO [5].
Atherosclerosis plays an important role in ischemia and the pathogenesis of various vascular diseases such as stroke and RVO, and macrophages and endothelial cells are major cellular constituents of atherosclerosis. A range of cell types, including macrophages and endothelial cells, produce PTX3 in response to inflammatory stimuli such as bacterial endotoxin, IL-6, and TNF. Additionally, elevated levels of systemic inflammatory cytokines such as IL-6 have been reported in RVO cases [5]. This relationship between atherosclerotic pathogenesis and PTX3 prompted the current study investigating an association between RVO and PTX3, and whether PTX3 might serve as a biomarker and a prognostic factor for RVO.
This study shows that patients with RVO have significantly elevated levels of PTX3 compared with unaffected controls, but the levels of PTX3 were not significantly different between the CRVO and BRVO groups. This finding might be explained by a similar degree of macular edema despite different disease classifications, which was not accounted for in this study. The existence of an undetected infection may also have contributed to a decreased sensitivity for a difference between patients with CRVO and BRVO. This study is limited by the small number of patients and the lack of information on other inflammatory markers like IL-6, TNF-α and myeloperoxidase, which precludes a comparison between the prognostic value of PTX3 and other inflammatory markers. Finally, because the PTX3 level was only measured once for each patient, individual variation could not be assessed.
Although there were some limitations in this study, these findings suggest that PTX3 can be used as a diagnostic biomarker of RVO. Further observational studies in various clinical situations with a larger number of participants will be needed to confirm these results and corroborate its usefulness as a prognostic biomarker. To consider a PTX3-lowering strategy would then be the next step.
REFERENCES
1. Shahid H, Hossain P, Amoaku WM. The management of retinal vein occlusion: is interventional ophthalmology the way forward? Br J Ophthalmol 2006;90:627-639.



2. Funk M, Kriechbaum K, Prager F, et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Invest Ophthalmol Vis Sci 2009;50:1025-1032.


3. Jo N, Wu GS, Rao NA. Upregulation of chemokine expression in the retinal vasculature in ischemia-reperfusion injury. Invest Ophthalmol Vis Sci 2003;44:4054-4060.


4. Pe'er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 1998;105:412-416.


5. Lee HB, Pulido JS, McCannel CA, Buettner H. Role of inflammation in retinal vein occlusion. Can J Ophthalmol 2007;42:131-133.


6. Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 2001;47:403-411.


7. Ridker PM, Cushman M, Stampfer MJ, et al. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 1998;97:425-428.


8. Goodman AR, Cardozo T, Abagyan R, et al. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev 1996;7:191-202.


9. Manfredi AA, Rovere-Querini P, Bottazzi B, et al. Pentraxins, humoral innate immunity and tissue injury. Curr Opin Immunol 2008;20:538-544.


10. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805-1812.



11. Noma H, Mimura T, Masahara H, Shimada K. Pentraxin 3 and other inflammatory factors in central retinal vein occlusion and macular edema. Retina 2014;34:352-359.


12. Bottazzi B, Vouret-Craviari V, Bastone A, et al. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem 1997;272:32817-32823.


13. Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol 2005;23:337-366.


14. Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro-inflammatory cytokines in acute coronary syndromes: from bench to bedside. Cytokine Growth Factor Rev 2006;17:225-233.


15. Alberti L, Gilardini L, Zulian A, et al. Expression of long pentraxin PTX3 in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis 2009;202:455-460.


16. Woo JM, Kwon MY, Shin DY, et al. Human retinal pigment epithelial cells express the long pentraxin PTX3. Mol Vis 2013;19:303-310.


17. Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 2001;21:1876-1890.


18. Deban L, Russo RC, Sironi M, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol 2010;11:328-334.



19. Souza DG, Amaral FA, Fagundes CT, et al. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am J Pathol 2009;174:1309-1318.



20. Foteinos G, Xu Q. Immune-mediated mechanisms of endothelial damage in atherosclerosis. Autoimmunity 2009;42:627-633.


21. Rolph MS, Zimmer S, Bottazzi B, et al. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2002;22:e10-e14.


22. Yu HI, Sheu WH, Song YM, et al. C-reactive protein and risk factors for peripheral vascular disease in subjects with Type 2 diabetes mellitus. Diabet Med 2004;21:336-341.


23. Kume N, Mitsuoka H, Hayashida K, Tanaka M. Pentraxin 3 as a biomarker for acute coronary syndrome: comparison with biomarkers for cardiac damage. J Cardiol 2011;58:38-45.


24. Lee DH, Jeon HK, You JH, et al. Pentraxin 3 as a novel marker predicting congestive heart failure in subjects with acute coronary syndrome. Korean Circ J 2010;40:370-376.



25. Jenny NS, Arnold AM, Kuller LH, et al. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 2009;29:594-599.


26. Peri G, Introna M, Corradi D, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 2000;102:636-641.


27. Akerfeldt T, Larsson A. Pentraxin 3 increase is much less pronounced than C-reactive protein increase after surgical procedures. Inflammation 2011;34:367-370.



28. Martin SC, Butcher A, Martin N, et al. Cardiovascular risk assessment in patients with retinal vein occlusion. Br J Ophthalmol 2002;86:774-776.






 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




