Macular edema including cystoid macular edema (CME) is one of the main causes of unfavorable visual outcomes after cataract surgery [
1,
2]. The incidence of clinically significant cases is 1% to 2%, whereas the incidence of angiographic cases is up to 9% to 19% [
3-
5]. In most cases, CME regresses spontaneously; however, macular thickness elevation persists in some cases and can lead to permanent visual deterioration [
3,
6,
7]. Increased central macular thickness (CMT) itself may also cause visual deterioration without intraretinal cystoid edema.
Diagnosis of macular edema previously depended on slit-lamp microscope examination and fundus fluorescein angiography; however, this approach is limited in its ability to obtain objective and quantitative data, and the interpretation of results varies between examiners. Recently, the introduction of optical coherent tomography (OCT) enabled morphologic and quantitative evaluation of macular status [
8-
10]. Several studies have reported the development of macular edema after cataract surgery [
9,
11-
13]. Most of these studies employed slit-lamp examination or fundus fluorescein angiography to assess the pathological changes in the macula. There are few large-scale studies focused on evaluation of serial macular changes after cataract surgery by using OCT images. Kim et al. [
9] reported the result of 50 cases using OCT; however, they only enrolled patients with diabetes mellitus (DM).
An observational study was designed in patients who underwent cataract surgery and aimed to evaluate macular thickness and morphological changes in the macula by using serial clinical examinations and OCT.
Materials and Methods
Ethics statement
In this retrospective observational study, 404 eyes of 404 patients underwent phacoemulsification and intraocular lens (IOL) implantation at the Department of Ophthalmology at The Catholic University of Korea. This study was performed in accordance with the tenets of the Declaration of Helsinki. The study protocol and informed consent were reviewed and approved by the Institutional Review Board of Seoul St. Mary’s Hospital (No. KC14RISI0191). Informed consent was waived due to the retrospective nature of the study.
Patient selection
We included patients who underwent cataract surgery to reduce visual impairment. Exclusion criteria were as follows: (1) known ocular diseases, such as corneal opacity, diabetic retinopathy, epiretinal membrane, age-related macular degeneration, and uveitis; (2) preexisting macular edema detected during preoperative evaluation; (3) invisible fundus or media opacity which can interfere with optimal imaging in OCT; (4) history of using prostaglandin analogues; and (5) intraoperative complications that could cause macular edema after surgery, such as posterior capsular rupture, damage of iris, iris protrusion through corneal incision site, vitreous loss, remaining lens cortex after surgery, posterior capsulotomy shortly after surgery, anterior chamber lens insertion, and IOL scleral fixation [
14]. Finally, 28 eyes of 28 patients were excluded, total 376 eyes of 376 patients were enrolled to the present study.
Preoperative examination
Preoperative data on patient demographics including age, gender, and general disease status such as DM or hypertension were collected. The preoperative ocular examination included best-corrected visual acuity (BCVA), manifest refraction, and slit-lamp (Huvitz, Anyang, Korea) evaluation including fundus photography. BCVA was measured using Early Treatment of Diabetes Retinopathy Study charts (Precision Vision, La Salle, Illinois) at a distance of 4 m under photopic conditions. Nuclear opacity of the lens was recorded according to the Lens Opacities Classification System III [
15] by using slit-lamp examination.
To determine IOL power during the cataract surgery, corneal thickness, anterior chamber depth, axial length, and corneal curvature were measured. Corneal thickness of each eye for all subjects were measured by Pentacam (Oculus, Wetzlar, Germany) and anterior chamber depth and axial length were measured by IOL Master (Carl Zeiss AG, Jena, Germany). Corneal curvature (keratometry) was also measured by automatic kerato-refractometer (Topcon, Tokyo, Japan). Additionally, macular thickness and OCT images were obtained with Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) to check for CMT and confirm the presence of macular disease or preexisting macular edema. OCT examinations were performed by experienced technicians. CMT was obtained from the thickness map scan protocol of Spectralis OCT. A raster horizontal 20° × 15°, 19-line scan, with a reciprocal distance of 240 mm, centered on the fovea (6 × 6-mm area) was collected. Only reliable OCT maps with image quality score more than 16 dB, were selected. Correct recognition of the retinal boundaries, namely vitreoretinal interface and retinal pigment epithelium and Bruch’s membrane junction, by the OCT automated segmentation software was checked on each scan. In this study, we defined CMT as the mean retinal thickness in the central area within 1-mm diameter displayed in the thickness map and no manual measurement were used in the analysis. In addition to CMT, the morphological changes in the macula such as cystoid formation or epiretinal membrane, were also assessed using OCT images by retinal specialists (SIC and MYL).
Surgical technique
All eyes were treated with topical antibiotics from 3 days before the cataract surgery. Tropicamide 0.5% and phenylephrine 0.5% (Mydrin-P; Santen, Osaka, Japan) were applied to dilate the pupil before the surgery. Cataract surgery was performed by a single surgeon (CKJ) under topical anesthesia using 0.5% proparacaine hydrochloride (Alcaine; Alcon Laboratories, Fort Worth, TX, USA). Clear corneal incision (2.75-mm to 3.0-mm in length) was created on the temporal side. After injecting viscoelastic material into the anterior chamber, a continuous curvilinear capsulorhexis of 5.0-mm to 6.0-mm diameter was created. Following hydrodissection and hydrodelineation, phacoemulsification was performed with Infiniti Vision System (Alcon Laboratories). Residual cortex material was aspirated using irrigation and aspiration probe and a foldable IOL was inserted in the capsular bag. In cases with constricted pupil (pupil diameter less than 5.0 mm) during surgery, epinephrine (0.5 mL, 1 : 100,000 solution) was injected into the anterior chamber with a hydration cannula in order to secure good visualization of the surgical field.
The intraoperative values or parameters were recorded to determine risk factors related to the occurrence of macular edema or CME. Total phaco time, phaco energy (cumulative dissociated energy), used balanced salt solution volume, prechopping, bottle height, and the use of intracameral epinephrine were assessed during the surgery. Postoperatively, topical antibiotics and steroid eye drops were used four times a day for 2 months after the surgery.
Postoperative examination
Patients visited Seoul St. Mary’s Hospital at 1 day, 1 week, 1 month, 2 months, and 6 months postsurgery and BCVA was examined during each visit. Spectral domain OCT images were obtained with Spectralis during each follow-up visit except at day 1 and month 6. Patients with elevated CMT postsurgery requiring treatment underwent additional OCT examination at 6 months postsurgery. Patients were classified into two groups (group 1, patients with no macular edema; group 2, patients with macular edema). Group 2 was defined in the present study as patients with >30% increase in CMT after uncomplicated cataract surgery based on the previous studies [
9,
12,
13]. Subgroups for group 2 were divided into two groups: patients with subclinical macular edema (group 2A) and CME (group 2B), respectively. Group 2A was defined as patients with the macular edema as an increase of center macular thickness (CMT) on OCT more than 30% from preoperative baseline and without a symptomatic drop in vision to worse than 20 / 40. Group 2B was defined as patients with a symptomatic drop in vision to worse than 20 / 40.
The risk factors for macular edema were evaluated by comparing group 2 to group 1. Other factors associated with preoperative and intraoperative parameters such as BCVA, total phaco time, and use of intracameral injection of epinephrine were also analyzed to confirm their association with macular edema.
Statistical analysis
This study was an observational only study. Therefore, we did not calculate the sample size based on the statistical power. Statistical analysis was performed using IBM SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Statistical analysis was performed using Wilcoxon rank-sum test on continuous variables and Fisher exact test on categorical variables for univariate analysis. One-way analysis of variance (ANOVA) was used to identify differences in BCVA or CMT between each of the patient groups. Then, post hoc test using Hochberg GT2 was performed, if there was a statistically significant difference between groups in ANOVA. A p-value of <0.05 was considered statistically significant. All values were presented as mean ± standard deviation or number (%), unless otherwise stated.
Results
A total of 376 eyes of 376 patients were enrolled in this study, of which 156 eyes (41.5%) were male patients and the mean age of subjects was 63.81 ± 11.82 years (range, 31-91 years). The average preoperative BCVA was 0.52 ± 0.40 (logarithm of the minimum angle of resolution, Log-MAR), and the average preoperative CMT was 194.96 ± 22.89 μm (
Table 1). All patients visited the clinic 1 day, 1 week, 1 month, 2 months, and 6 months postsurgery. No significant complications that might result in elevated CMT or be related to the cataract surgery were observed during the follow-up period.
CMT and morphological changes were evaluated by examining OCT images. All subjects were divided into group 1 and 2 according to the CMT after the surgery. Group 2 was defined as 30% increase of CMT compared with that before surgery. Thirty-six patients (9.6%, group 2) developed macular edema after surgery, as assessed by OCT.
Univariate analysis for group 1 and 2 revealed that intracameral injection of epinephrine during phacoemulsification was associated with the development of macular edema (
Table 2). There was no statistically significant difference in other values or parameters including preoperative and intraoperative measurements (
p > 0.05).
In group 2, five patients (1.3%, group 2B) developed CME. Univariate analysis did not reveal any statistically significant differences in other parameters; this analysis was performed by dividing group 2 into group 2A and 2B according to the presence of CME (
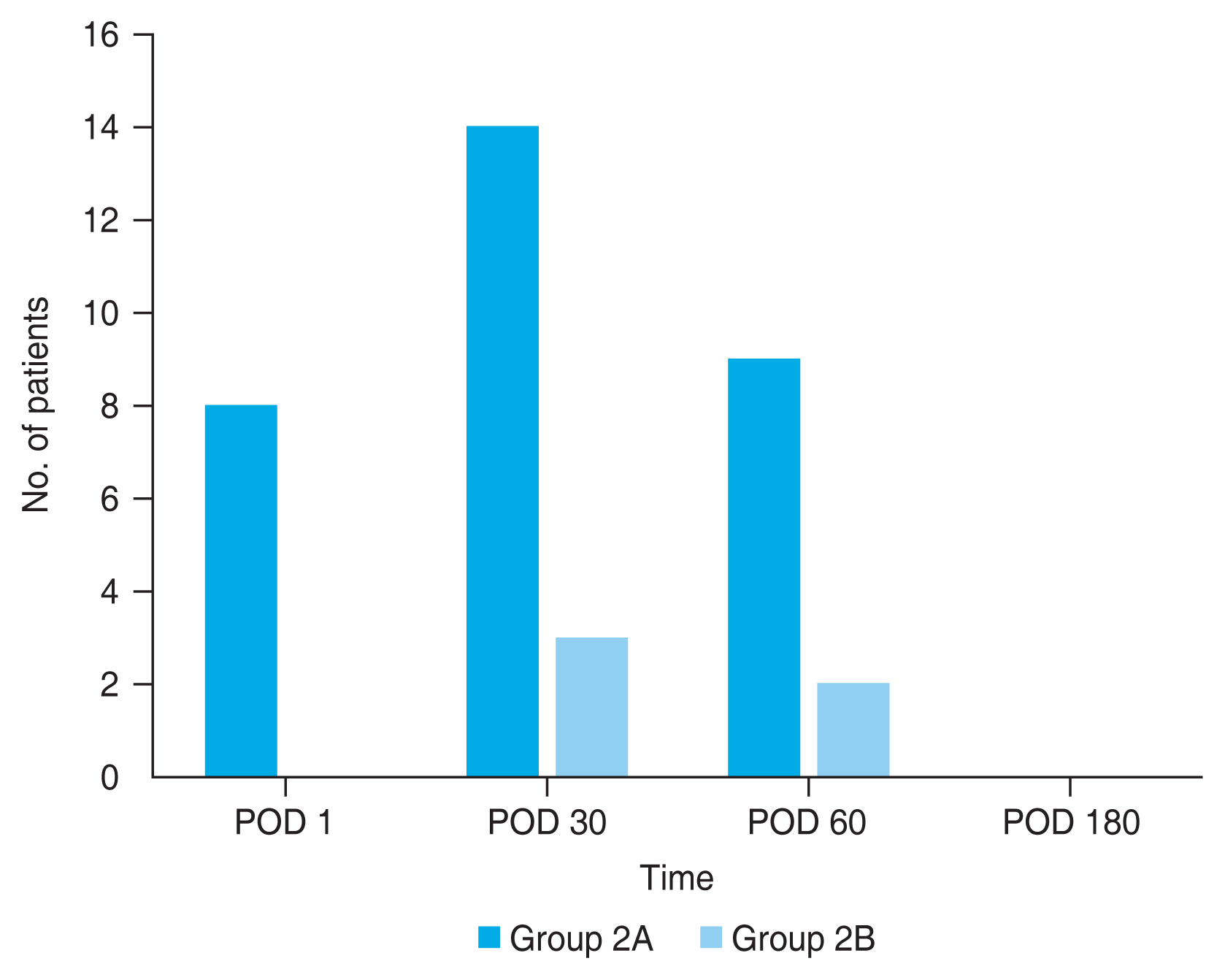
Table 3). Macular edema was found in eight (22.2%), 17 (47.2%), and 11 patients (30.6%) in group 2 at 1 week, 1 month, and 2 months postsurgery, respectively (
Fig. 1).
Fig. 1 also showed that macular edema at 1 month postsurgery had the most cases of macular edema in both group 2A (14 of 31 patients, 45.2%) and 2B (three of five patients, 60.0%).
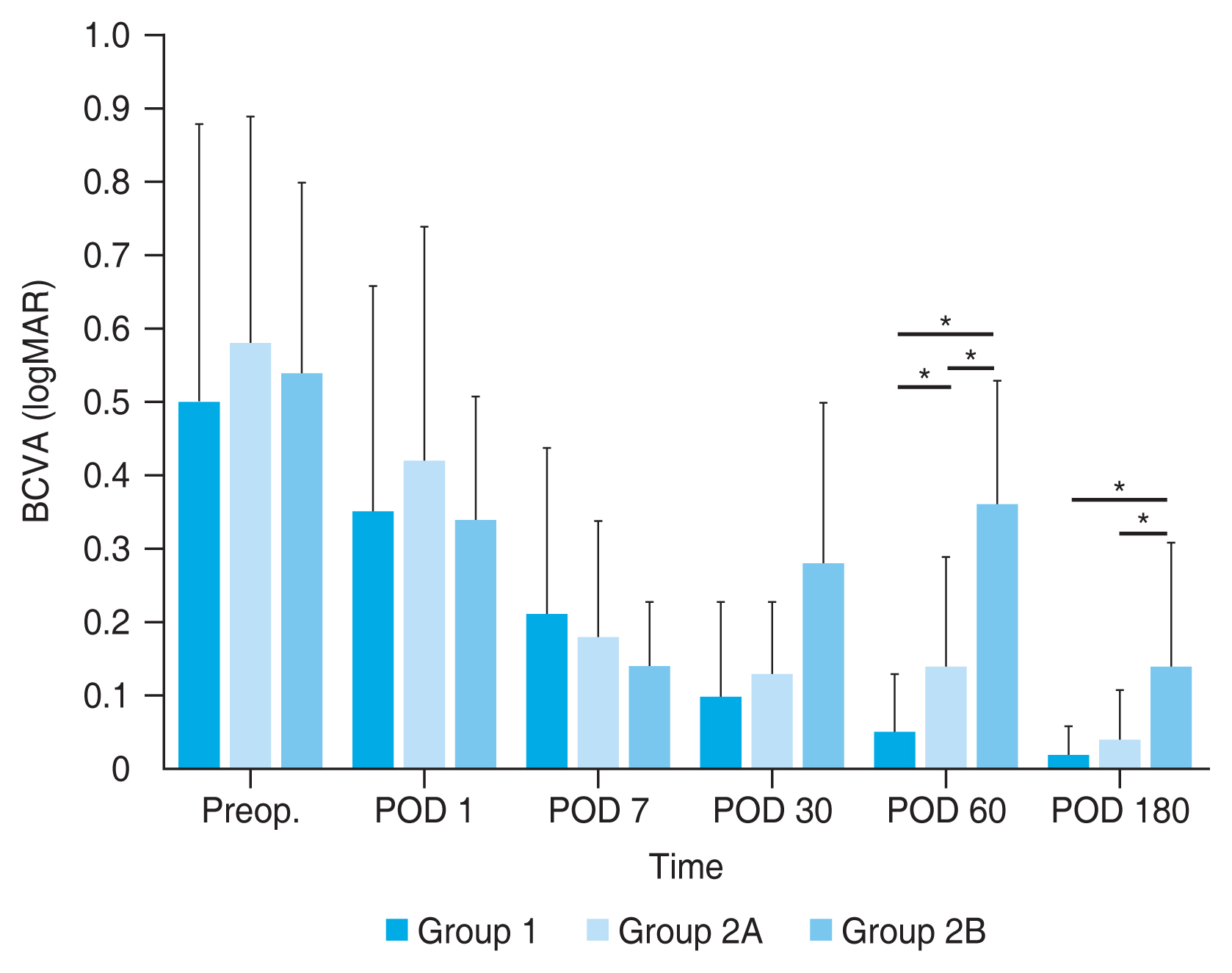
Preoperative CMT was 195.4 ± 22.5, 189.2 ± 27.8, and 198.6 ± 13.7 μm in group 1, 2A, and 2B, respectively, and there was no difference between the three groups (
Fig. 2). Group 1 (
p < 0.001) and group 2A (
p < 0.001) showed better BCVA at 2 and 6 months postsurgery and lower CMT at 1 month postsurgery (198.5 ± 23.6 and 237.8 ± 40.9 μm in group 1 and 2A, respectively) and 2 months postsurgery (201.2 ± 23.1 and 250.0 ± 30.0 μm in group 1 and 2A, respectively) compared to those in group 2B (314.0 ± 104.5 and 371.0 ± 160.3 μm at 1 and 2 months postsurgery, respectively). At 2 months postsurgery, BCVA in group 2A (
p < 0.001) was better and CMT in group 2A (
p < 0.001) was lower than those in group 2B (
Fig. 2,
3).
Fig. 2 also revealed that CMT in group 1 at 1 month postsurgery (
p < 0.001) was lower than those in group 2A, but there was no statistically significant difference in BCVA between those groups at that time point (
p > 0.05).
Group 2B required additional treatment for CME. Of five eyes, four eyes were treated with intracameral injection of epinephrine during the surgery. They were treated with a topical nonsteroidal anti-inflammatory drug (NSAID) and a topical corticosteroid (Pred Forte; Allergan Inc., Irvine, CA, USA) in combination with subtenon triamcinolone acetonide injection (Triamcinolone; Dongkwang Pharmaceutical Co., Seoul, Korea) or intravitreal Avastin injection (bevacizumab; Genentech Inc., South San Francisco, CA, USA).
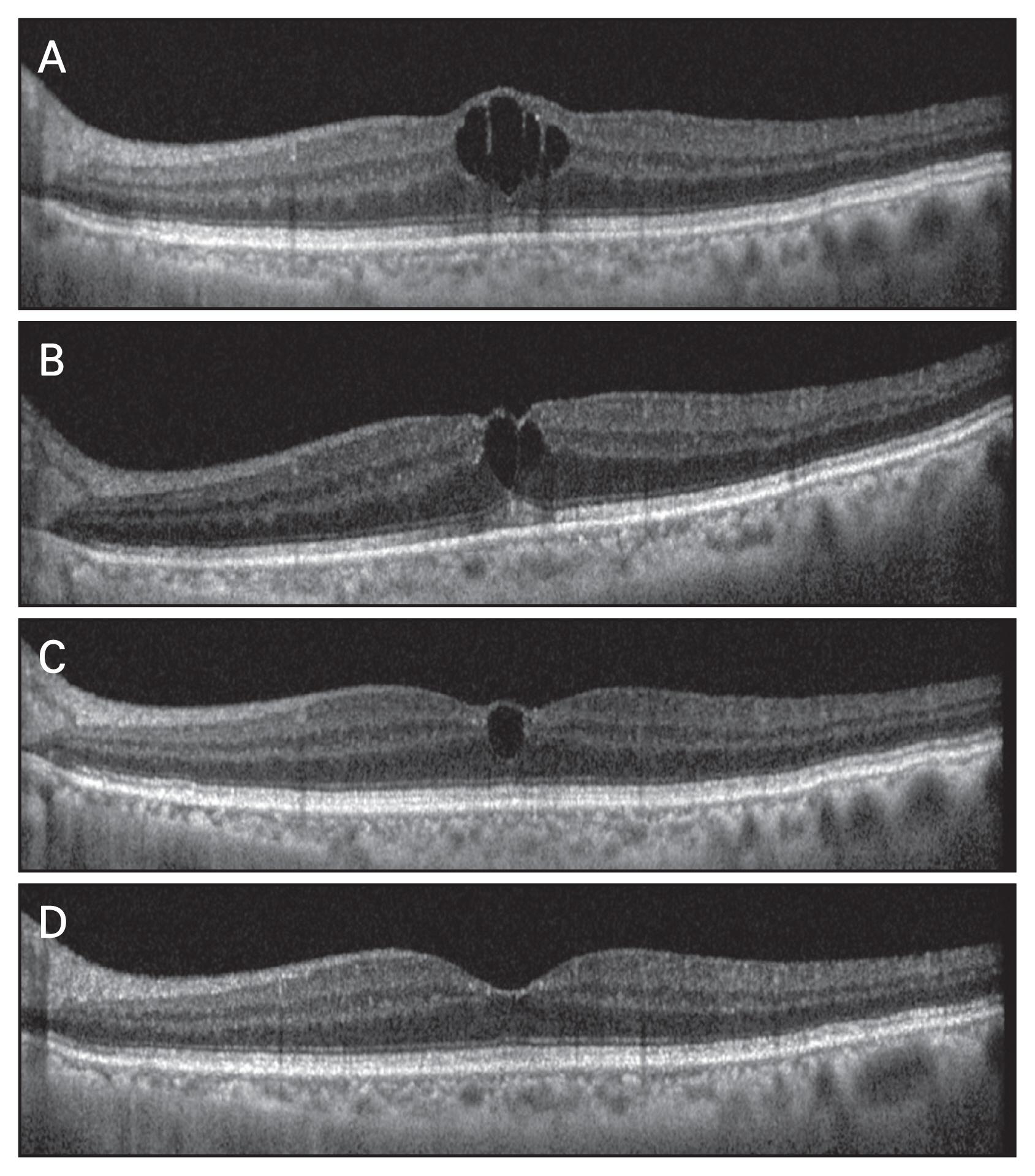
Except for one eye, four eyes achieved BCVA of 0.2 (LogMAR) or better with CMT in the normal range at 6 months postsurgery (
Table 4). The exceptional case was that of an 82-year-old male patient with no history of systemic or ocular disease. Increased CMT with cystic changes at the fovea was detected by examining the OCT image at 1 month after the surgery. Then, the patient received intravitreal injection of Avastin in combination with a topical NSAID and a topical corticosteroid. In spite of the treatment, CME was persistent for 6 months after the surgery. He received second intravitreal injection of Avastin in combination with the use of a topical NSAID and a topical corticosteroid at 6 months postsurgery. The CMT finally stabilized to 232 μm and intraretinal cyst disappeared at 7 months postsurgery (
Fig. 4A-4D). BCVA also recovered to 0.1 during that time.
Discussion
In this retrospective observational study, we objectively evaluated the macular thickness and the occurrence of macular edema after uncomplicated cataract surgery using OCT. OCT has already been reported as a reliable tool for evaluating macular thickness in normal population and patients with DM, with high reproducibility [
16,
17]. There have been many reports on macular edema after cataract surgery. However, to the best of our knowledge, there was no large-scale study to objectively investigate the frequency and the clinical course of increased CMT after uncomplicated cataract surgery using OCT.
In general, according to the previous studies, the incidence of clinically significant cases was 1% to 2%, whereas the incidence of angiographic cases was up to 9% to 19% [
3-
5]. Mentes et al. [
18] reported that the incidence of clinical and angiographic CME in patients without specific underlying conditions was 0% and 9.1%, respectively. Although they assessed macular thickness using fundus fluorescein angiography, the results of their study were in agreement with those of our study.
The majority of symptoms and signs of clinically significant increase in CMT appear 4 to 12 weeks postsurgery, with peak incidence typically occurring between 4 to 6 weeks [
14,
19]. Eyes with subclinical macular edema or CME had elevated CMT compared to those with no macular edema at 1 month postsurgery in our study. However, the difference in CMT did not appear between no macular edema and subclinical macular edema group at that time point. We hypothesized that changes in CMT could be examined in detail and more accurately by using OCT rather than by using BCVA. In other words, OCT images can provide better information about macular status after cataract surgery than BCVA.
A previous study using an animal model to investigate the pathophysiology of the retina in myopia using vitreous fluorophotometry revealed that the blood-retinal barrier in myopia is abnormal. The inward permeability significantly increased, and the outward permeability decreased compared to that of emmetropic eyes. This suggested that the active transport mechanism at the blood-retinal barrier decreases in myopia [
20]. However, contrary to expectations that myopic eyes will be prone to postoperative CMT augmentation, there was no significant correlation between axial length and CMT. Giansanti et al. [
21] also reported that the incidence of CMT elevation does not increase in patients with severe myopia after uncomplicated cataract surgery. Lowering the bottle height during phacoemulsification in order to prevent excessive anterior chamber deepening, which might cause posterior capsular rupture and vitreous protrusion, may have biased the results of our study. The only factor that affected macular thickness was the intracameral injection of epinephrine during the surgery.
Epinephrine is widely used as a medication for glaucoma or as a mydriatic agent and is known to contribute to elevated macular thickness, especially when applied to aphakic eyes [
22]. Although pupil dilation is maintained with preoperative topical mydriatic eye drops, at times, additional intracameral injection of epinephrine is required since secured visualization of a surgical field is crucial for proper outcome of surgery. Bozkurt et al. [
23] reported that intracameral injection of epinephrine (1 : 5,000) does not increase the risk of macular edema due to CMT elevation in eyes with no risk factors in the context of uneventful phacoemulsification with IOL implantation. However, bisulfite-free epinephrine was used in their study. Considering the current shortage of bisulfite-free epinephrine in South Korea as well as in the United States [
24], the effect of epinephrine with bisulfite should be reappraised.
Surgeons have no other choice but to use epinephrine-containing bisulfite owing to the absence of available bisulfite-free epinephrine in South Korea. Therefore, intracameral epinephrine with bisulfite (1 : 100,000) was employed in our study when there was a need for pupil dilatation during surgery. The CMT of patients who received epinephrine with bisulfite increased compared with those who did not receive intraoperative epinephrine injection throughout the follow-up period. Interestingly, 16 of 25 eyes (64.0%) that received intracameral injection of epinephrine during the surgery had elevated CMT at 1 month postsurgery, whereas 8 of 11 eyes (72.7%) that did not receive epinephrine injection had elevated CMT at 2 months postsurgery (not described in the main manuscript of this study). The time to onset of elevated CMT was earlier in the epinephrine group than that in the nonepinephrine group.
The earlier onset and the increased treatment demand owing to elevated CMT for the epinephrine group can be explained (
Table 4). One is the possibility that the adrenergic effect of epinephrine mediates the dilatation of retinal vessels increasing the blood flow in the central macula. The other is that epinephrine acts as a cofactor for the synthesis and release of prostaglandins, which can induce arteriolar vasodilation by iontophoresis [
25]. Another possibility is that the sodium bisulfite contained in commercially utilized epinephrine, which is known to have vasorelaxation effect, alters the blood-aqueous barrier function [
26]. The reason of relatively high percentage of using intraoperative epinephrine in group 1 might be due to the characteristics of patient group in our clinic. The cataract patients with small pupil had been often referred to the tertiary hospital clinic.
In this study, we obtained quantitative data regarding CMT using OCT after uncomplicated cataract surgery and found that intraoperative intracameral injection of epinephrine was a risk factor for elevated CMT. The final visual acuity and CMT in most patients with macular edema, including subclinical macular edema, normalized postsurgery without any treatment. CME cases were objectively followed up until 6 months postsurgery using OCT to evaluate the clinical course of CME and the final visual outcomes. The overall visual outcome at 6 months after the operation was favorable. Therefore, surgeons should be aware of the complications of intracameral injection of epinephrine and should not abuse it unnecessarily. It is recommended to examine CMT using OCT for detection of macular edema, even if the cataract surgery was performed without any intraoperative complications.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



