Among the conventional steps for cataract surgery, continuous curvilinear capsulotomy is an important procedure that can influence subsequent steps such as hydrodissection, phacoemulsification of the lens, and insertion of the intraocular lens (IOL) [1]. Precise and proper size of capsulotomy is crucial; this is because small capsulorhexis results in excessive anterior capsular fibrosis, regarded as residual lens epithelial cells [2], which could move the IOL backward to induce hyperopia [3,4]. Conversely, a large capsulorhexis can lead to IOL tilting, decentration, and early posterior capsule opacification [5-7].
In fact, it is impossible to manually create a uniform capsulotomy of the same shape and size. However, femtosecond laser-assisted cataract surgery (FLACS) can compensate for this shortcoming. FLACS can produce consistent and accurate anterior capsulotomy with high reproducibility and predictability [8-12]. Consistent and identical anterior capsulotomy can guarantee the precise positioning of the IOL [10]. However, FLACS requires numerous equipment, high procedure costs, longer operating times, and additional space. Additionally, it may be difficult to perform femtosecond laser procedures in patients with poor mydriasis or corneal opacity [13].
The recently introduced precision pulse capsulotomy (PPC) (Zepto; Mynosys, Fremont, CA, USA) is a semi-automated alternative to conventional capsulotomy. Using 12 micropulses of energy in 4-ms intervals, PPC can instantly create a circular anterior capsulotomy with an average diameter of 5.2 mm [14]. In addition to our previous study [15], recent studies demonstrated that this thermal capsulotomy device produces consistent and reproducible symmetric capsulotomies [16-19]. According to the manufacturer, the temperature rise during thermal capsulotomy using Zepto PPC is not significant enough to damage the eyes, but no experimental or clinical studies confirming the heat generation by Zepto have been reported. Furthermore, in terms of devices that are based on heat generation, there is a possibility that they may cause serious damage to the intraocular tissues such as the endothelium or iris, as well as long-term complications due to capsular contraction. In this study, we aimed to elucidate the thermal safety of Zepto PPC by performing in vivo and in vitro evaluations of the thermal profile using infrared thermography during thermal capsulotomy.
Materials and Methods
Patients
Of the patients scheduled for cataract surgery, we enrolled 15 patients (15 eyes) who underwent cataract surgery in this prospective observational study from May 2018 to October 2018. Seven eyes on the right and eight eyes on the left were included from seven male and eight female patients. The research was performed at the department of ophthalmology, Dongsan Medical Center, affiliated with Keimyung University in Daegu, Korea. All clinical evaluations and measurements followed the tenets of the Declaration of Helsinki, and the research protocol was reviewed and approved by the Ethics Committee of Dongsan Medical Center (DSMC 2018-07-007). Informed consent was obtained from all patients after describing the details of the study. The inclusion criteria were age-related cataracts with favorable physical status and uneventful in-the-bag IOL implantations of one of both eyes. Those with a previous history of intraocular surgery, previous ocular trauma or uveitis, an axial length shorter than 22.0 mm or longer than 24.5 mm, pseudoexfoliation syndrome, poor pupil dilation, preoperative or perioperative zonular weakening were excluded.
Measurements
Ophthalmologic examinations included several parameters. The preoperative values were axial length and anterior chamber depth acquired using optical low-coherence reflectometry (Lenstar LS900; Haag-Streit AG, Bern, Switzerland). Cataract nuclear opalescence was graded from 1 to 5 according to the Lens Opacity Classification System III criteria [20]. Preoperative and 1-day postoperative values included uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA) recorded in logarithm of the minimum angle of resolution units, spherical equivalent performed by autorefraction (RK-F2; Canon, Tokyo, Japan), and intraocular pressure (IOP) measured using a non-contact tonometer (NT-530P; Nidek, Tokyo, Japan).
Surgical procedures
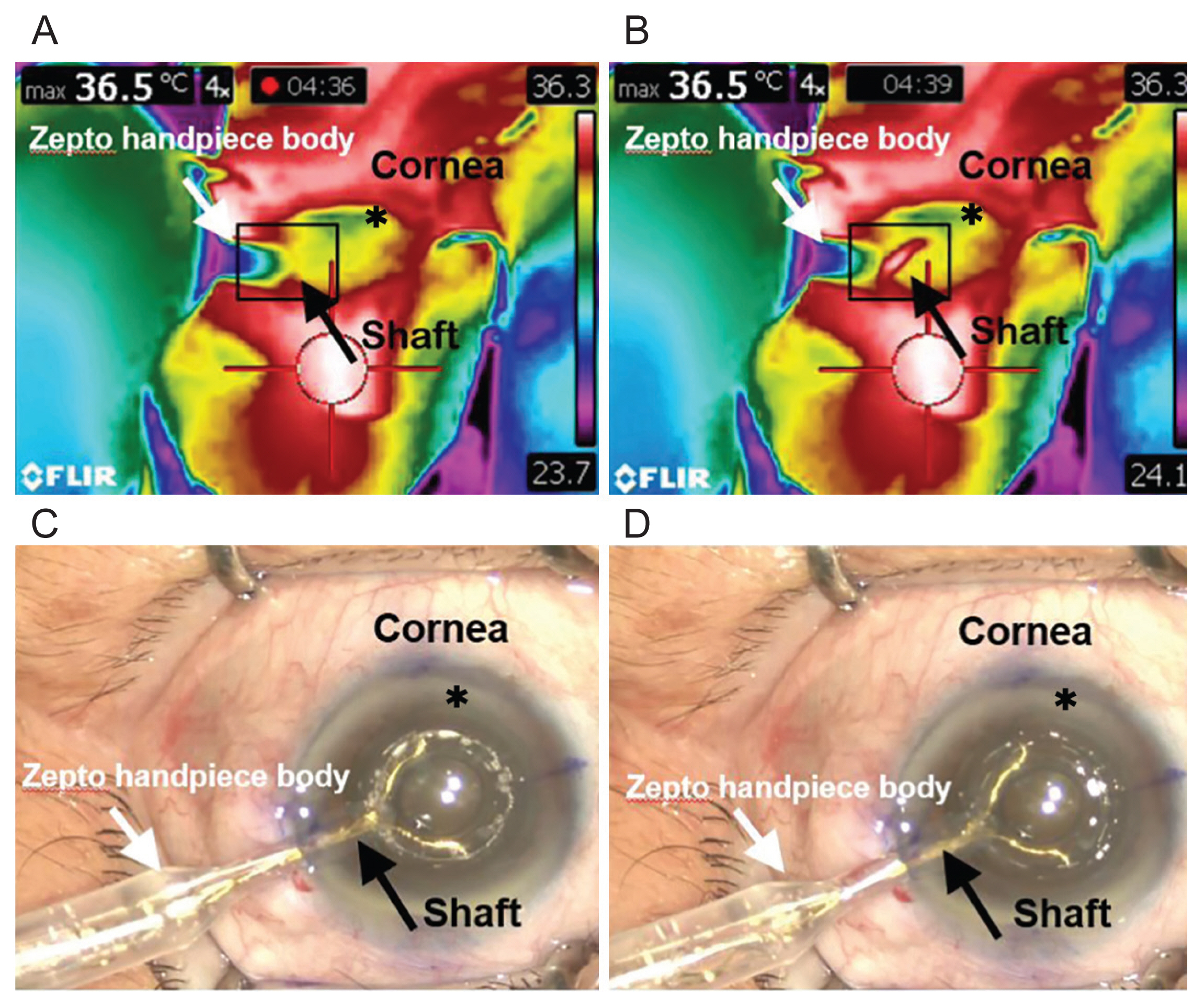
Approximately 1 hour before surgery, each patient was twice-instilled with 0.5% tropicamide/0.5% phenylephrine fixed combination eye drops (Tropherine; Hanmi Pharm, Seoul, Korea) for 5 minutes in order to maximize pupil dilation. All surgeries were performed by the same surgeon (JHJ), who performed a 2.85 coaxial incision (Infiniti Vision System; Alcon, Fort Worth, TX, USA). After instillation of 0.5% proparacaine topical anesthetic eye drops (Paracaine, Hanmi Pharm), a clear corneal incision was made at the 9 oŌĆÖclock (right eye) and 2 oŌĆÖclock (left eye) positions. Before anterior capsulotomy, a viscoelastic device (DisCoVisc, Alcon) was placed in the anterior chamber through a sideport incision at the 12 oŌĆÖ clock position (right eye) and 5 oŌĆÖ clock position (left eye). During the capsulotomy procedure, we performed corneal marking using a 5.5-mm capsulotomy marker. The PPC deviceŌĆÖs nitinol cutting element was inserted into the anterior chamber through the corneal incision and positioned centrally on the anterior capsule under the corneal marking. An assistant then applied suction to allow for the apposition of the nitinol ring with the capsule. After adequate suction was completed, the cutting procedure was performed, as indicated by the console, and the electrical energy was released. After the introduction of the Zepto PPC handpiece, the thermal profile of the corneal incision site adjacent to the Zepto PPC shaft was recorded using an infrared thermographer (T420bx; Flir Systems, Wilsonville, OR, USA) with a temperature range of ŌłÆ20┬░C to 350┬░C and thermal sensitivity of 0.04┬░C (Fig. 1A-1D). All thermographic profiles were recorded as sequence files on a connected laptop computer and analyzed using the Flir tool + (Flir Systems). To easily rotate the lens nucleus, hydrodissection was conducted, and phaco-chop was performed to emulsify and remove the nucleus. After removal of the remaining cortex material, insertion of the hydrophobic one-piece IOL (AcrySof IQ SN60WF, Alcon), and aspiration of the ophthalmic viscosurgical device (OVD), the corneal wound was hydrated. Patients were treated with 0.5% moxifloxacin eye drops (Vigamox, Alcon) and a 1% prednisolone acetate ophthalmic suspension (PredForte; Allergan, Irvine, CA, USA) four times a day after surgery for more than 3 weeks.
In vitro experimental procedures
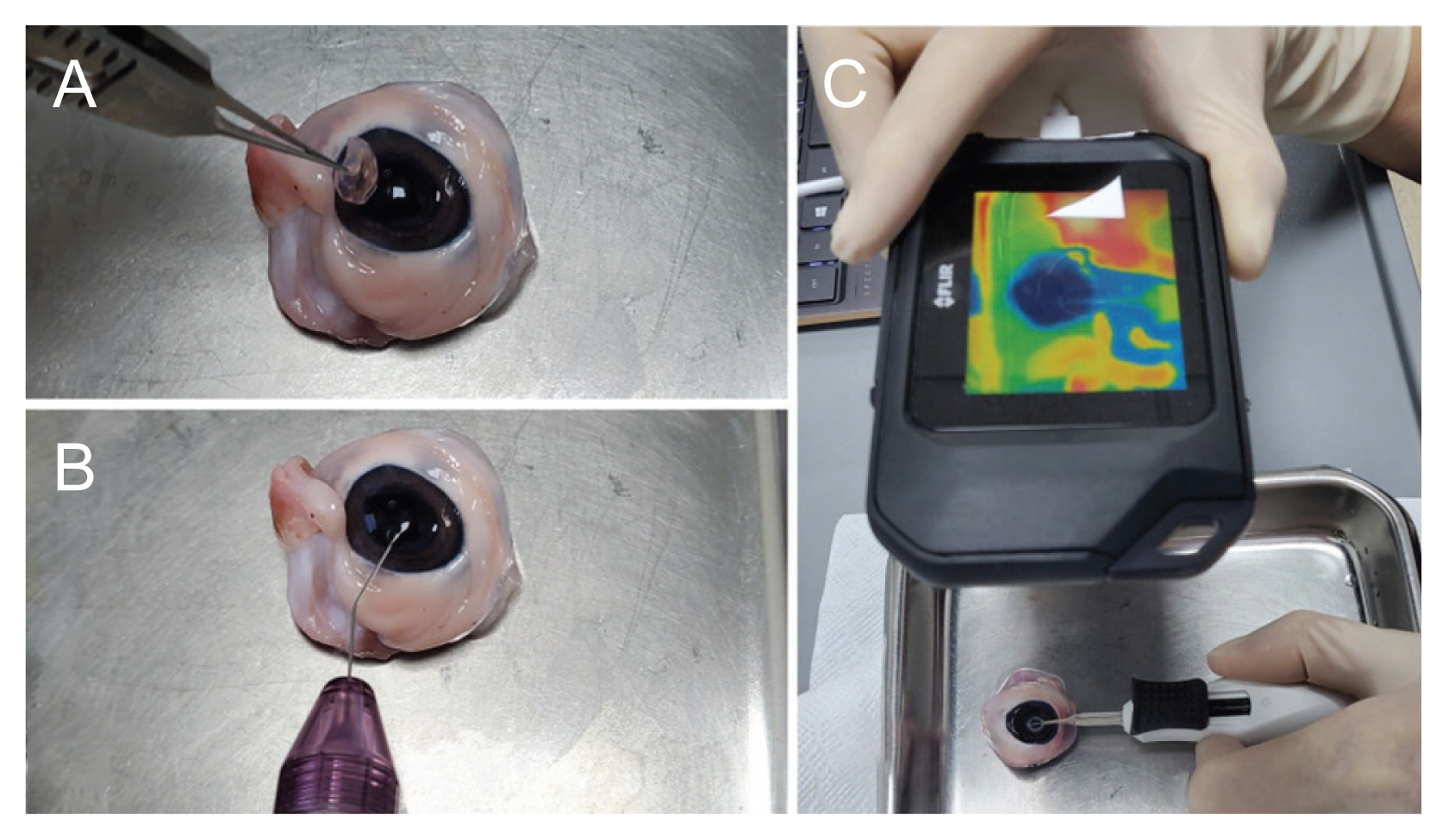
Porcine eyes were obtained from a slaughterhouse approximately 3 to 5 hours after death. An in vitro study was carried out to record temperature using three conditions: (1) with the Zepto PPC nitinol ring positioned na├»vely in open air or on the porcine lens surface, (2) with, or (3) without an OVD. During the procedures, the thermal profile was recorded by directly targeting the ring using a compact infrared thermographer (Flir C2, Flir Systems) with a temperature range of ŌłÆ10┬░C to 150┬░C and a thermal sensitivity of 0.1┬░C. All thermographic profiles were recorded and analyzed using the Flir tool +. To check the na├»ve temperature, the Zepto PPC handle was fixed using silk tape on the floor with nothing around the Zepto PPC nitinol ring, and the thermal profile was acquired during the suction and cutting procedures in the top, lateral, and bottom views. To keep the measurement conditions similar to the in vivo experiment in which the OVD could be placed in the anterior chamber, the porcine cornea was resected above the limbus in a dome shape. The lenticular surface was coated with a DiscoVisc OVD. The Zepto PPC nitinol ring was positioned at the center of the porcine lens surface. Suction was applied, and the cutting procedure was performed in the same manner as for the human eye (Fig. 2A-2C). To identify the possible heat damage to intraocular tissues such as the iris, endothelium, and corneal incision according to the presence or absence of the OVD, thermal profiles were obtained in the top and lateral views. However, the thermal profile of the bottom side was excluded because the Zepto nitinol ring directly contacted the lens capsule and tissues. Each experiment was repeated at least three times.
Results
Patient demographics
The mean age of the patients was 67.9 ┬▒ 9.7 years; nine patients (60%) had hypertension and seven (47%) had diabetes. The mean nuclear opalescence grade was 3.40 ┬▒ 0.91, and the mean axial length and anterior chamber depths were 22.92 ┬▒ 0.77 and 3.18 ┬▒ 0.48 mm, respectively. The mean preoperative UCVA and BCVA were 0.49 ┬▒ 0.33 and 0.42 ┬▒ 0.34, respectively, and the mean postoperative UCVA and BCVA were 0.22 ┬▒ 0.17 and 0.12 ┬▒ 0.12, respectively. The mean preoperative IOP was 12.9 ┬▒ 1.8 mmHg and the mean postoperative IOP was 15.1 ┬▒ 3.9 mmHg. Both UCVA and BCVA improved significantly (difference: UCVA, 0.27; p = 0.009; BCVA, 0.30; p = 0.003).
In vivo thermal profile
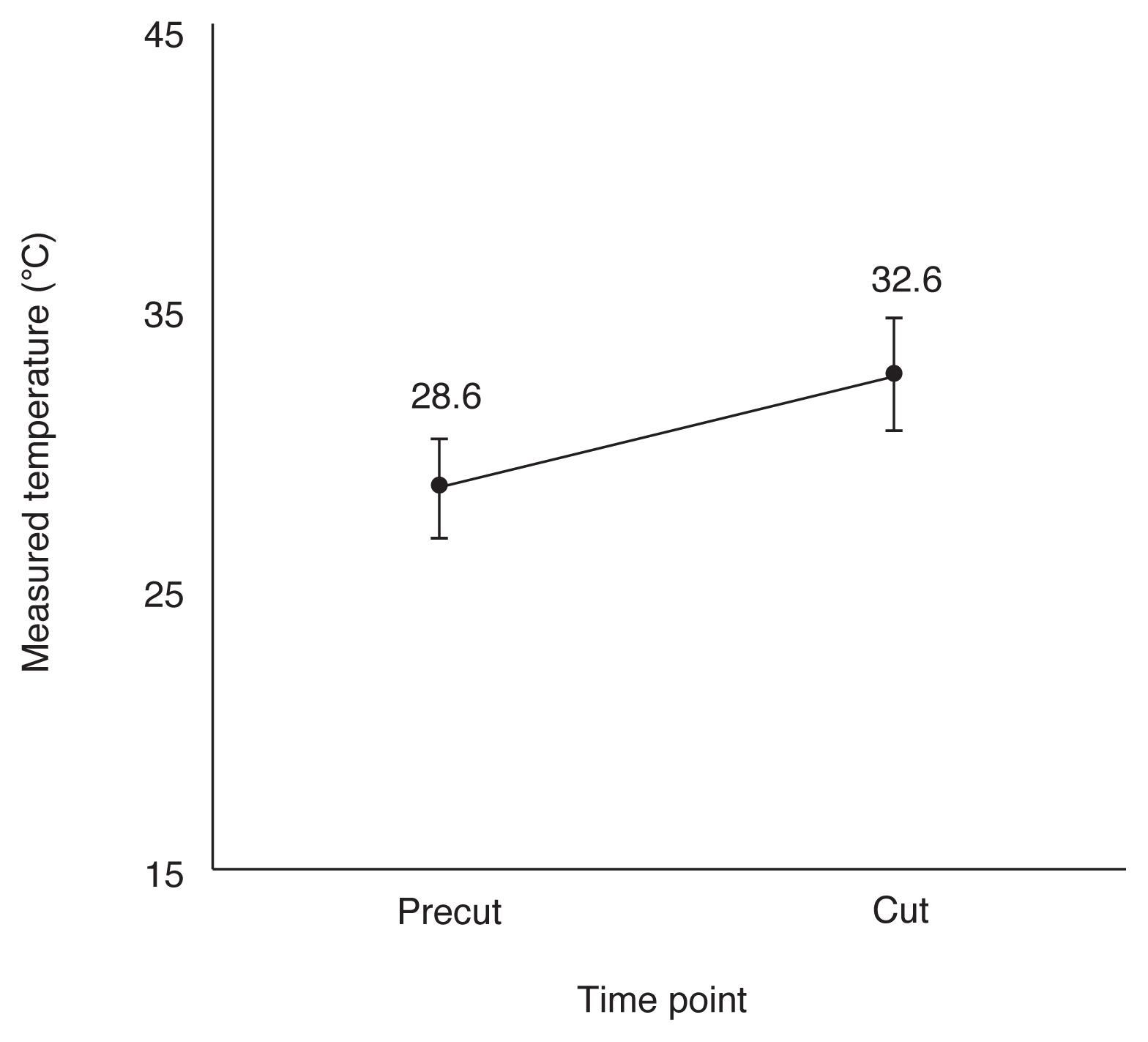
During the cutting procedure, the mean time to peak temperature after the temperature rose around the corneal incision was identified to be 4.43 ┬▒ 1.26 seconds (range, 1.93-6.57 seconds). Baseline and peak temperature were 28.6┬░C ┬▒ 1.7┬░C and 32.6┬░C ┬▒ 2.0┬░C (range, 29.2┬░C-37.2┬░C), respectively, which was insignificant (difference, 4.0┬░C ┬▒ 1.9┬░C; p = 0.063) (Fig. 3). The mean temperature rise ranged from 0.9┬░C to 7.2┬░C. The peak temperature was >30┬░C in 14 of 15 cases. The in vivo thermal profiles are presented in Table 1.
In vitro thermal profile
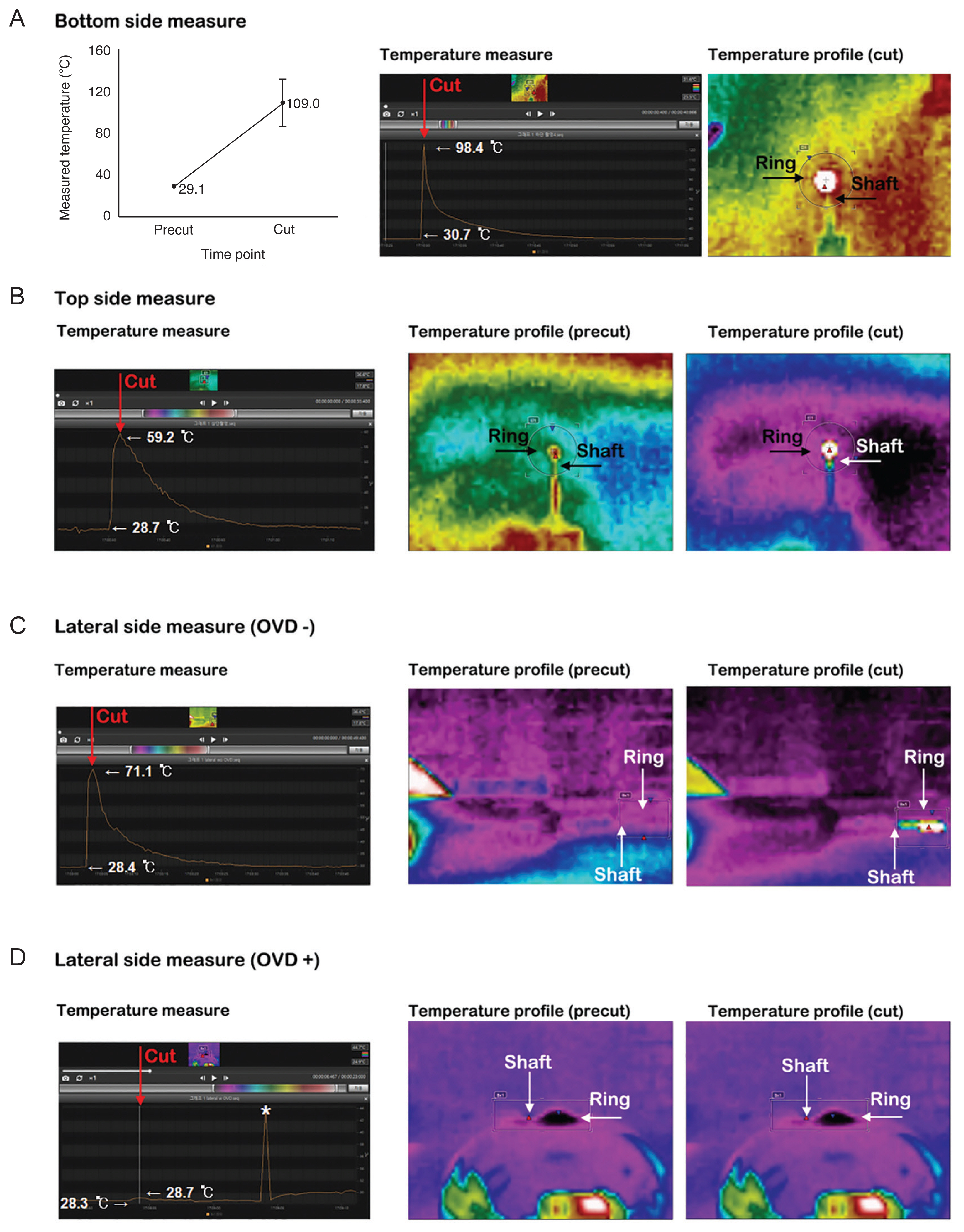
The temperature elevation of the Zepto nitinol ring was highest on the bottom side (Fig. 4A) among the top (Fig. 4B), bottom, and lateral views in the na├»ve cutting procedures. The mean temperature rise time of the bottom view was identified as 43.40 ┬▒ 11.06 seconds, and the mean temperature rise was 79.9┬░C ┬▒ 22.5┬░C. The baseline and peak temperatures were 29.1┬░C ┬▒ 1.0┬░C and 109.0┬░C ┬▒ 22.9┬░C, respectively (Fig. 4A). In the top view, the baseline temperature was similar with the bottom view, but the peak temperature was measured only half from the bottomŌĆÖs value (Fig. 4B). When viewed through a thermographer, the temperature change of the Zepto shaft was significantly different depending on the presence or absence of the OVD in the lateral view. Without the OVD, the shaftŌĆÖs color change was easily detectable; however, with it, the shaftŌĆÖs color change was minimal and difficult to recognize (Fig. 4C, 4D). In the top view, the mean temperature rise time was 14.00 ┬▒ 3.61 seconds, and the mean temperature rise was 10.5┬░C ┬▒ 3.3┬░C without the OVD. The baseline and peak temperatures were 18.2┬░C ┬▒ 0.1┬░C and 28.7┬░C ┬▒ 3.4┬░C, respectively (Fig. 5A). The mean temperature rise time of the Zepto nitinol ring with the OVD in the top view was 11.67 ┬▒ 2.08 seconds and the mean temperature rise was 6.2┬░C ┬▒ 1.6┬░C. The baseline and peak temperatures were 19.7┬░C ┬▒ 0.8┬░C and 25.9┬░C ┬▒ 2.3┬░C, respectively (Fig. 5B).
Discussion
In the present study, we evaluated the temperature profiles of the Zepto PPC device in vivo and in vitro. Previous studies on the Zepto PPC showed that the device is as safe as conventional phacoemulsification cataract surgery with respect to complications such as corneal edema, anterior chamber inflammatory reaction, capsular fibrosis, anterior capsular opacity, posterior capsular opacity, and endothelial cell loss [14,18,19]. In our previous 59-case prospective study, we also verified the safety of PPC compared with conventional cataract surgery at 6 months follow-ups; there, we demonstrated less effective lens position variability and better circularity in the PPC group than in the continuous curvilinear capsulotomy group [15]. However, the aforementioned studies did not assess the temperature of the corneal incision site or anterior chamber during the cutting procedure during the clinical surgeries, which could potentially lead to overheat damage at the endothelium and anterior capsule cut edge.
According to a study of 20 live rabbit eyes, the Zepto PPC device can increase the anterior chamber temperature by approximately 1°C to 2°C in the eye (maximum, 2.01°C) and return to the baseline temperature in 2 to 4 seconds (maximum, 4.60 seconds) [14]. In that particular study, the temperature was recorded using thermocouple temperature measurements, with probes placed in two locations in the anterior chamber adjacent to the suction cup and endothelium. However, our present study recorded general thermal profiles using an infrared thermographer; during the live human patient surgeries, the mean temperature elevation in the corneal incision site was 3.98°C ± 1.91°C, which was higher than 2.01°C. Moreover, in the in vitro study, the mean peak temperature in the naïve circumstance was elevated to 109.0°C ± 22.9°C during the cutting procedure, in the bottom view, with no media around the Zepto nitinol ring. In comparison to previous animal studies, the results from the thermal detection device differed from the present study. An animal study used thermocouple temperature measurements, and our study used an infrared thermographer to evaluate detailed thermal changes. Therefore, it is difficult to objectively compare the results of the two studies. However, Innocenti et al. [21] compared the difference in the measured temperature between a thermal camera and thermocouple during phacoemulsification and found that the difference between the two devices was only an average of 0.5°C. From our measurements, the temperature profiles of the naïve nitinol ring were inconceivably higher than those of previous animal studies (109.0°C ± 22.9°C).
In our previous study, cataract surgery using Zepto PPC caused minimal endothelial cell loss compared to conventional cataract surgery [15]. We hypothesized that an approximately 2┬░C to 6┬░C temperature elevation with a transient moment of approximately 3 to 6 seconds is not sufficient to influence the endothelium. Furthermore, in an in vitro study, the Zepto nitinol ring temperature elevation during contact with the Zepto nitinol ring on the porcine lens was much lower than that in the na├»ve status. This may have been due to effects from the media, such as the aqueous humor, anterior capsule, or particularly the OVD, which blocks and buffers the overheat damage from the corneal endothelium. When comparing the presence of the OVD in the cutting procedure, the OVD groupŌĆÖs mean temperature elevation was much lower and their elevation time longer than in the non-OVD group (Fig. 5). Previous in vitro studies of porcine eyes showed that during ultrasound oscillation, especially torsional vibration in phacoemulsification, there was little corneal endothelium temperature elevation in the OVD group compared to the balanced salt solution group [22]. Therefore, it is estimated that the temperature rise of the Zepto PPC will have a negligible effect on the intraocular tissue in a general cataract surgery environment.
A limitation of this study is that we did not use a thermal probe; therefore, we could not assess the anterior chamber or endothelium temperature. The thermal profiles were set using an infrared thermographer; therefore, in the in vivo study, the available temperature values were the corneal surface and corneal incision around the Zepto handpiece. In the case of the corneal surface, the essential temperature change was not clear due to irrigation of balanced salt solution and structures below the corneal surface (cornea, anterior chamber, and OVD). Therefore, measuring the temperature of the Zepto handpiece was the best way to assess the temperature change of the Zepto nitinol ring. However, in the in vitro study, since we removed the central 8.0-mm-diameter porcine cornea, we could assess the temperature change of the nitinol ring directly. From these results, we can predict the endothelial temperature indirectly.
In conclusion, our results suggest that the Zepto PPC nitinol ring itself could induce an extremely high thermal rise of approximately 100┬░C for capsulotomy during the cutting procedure. However, in the in vivo study, the temperature difference was minimal. In the actual use of this device, there are several surrounding tissues and buffers, including the anterior capsule, anterior chamber, and the OVD around the nitinol ring, that could prevent extreme temperature rises and alleviate overheat damage to nearby intraocular tissues. The in vitro analysis showed that the OVD offered excellent buffering effects by blocking the thermal energy. This would protect the corneal endothelium and cut edge of the anterior capsule from the risk of endothelial cell loss and capsular phimosis.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






