|
 |
| Korean J Ophthalmol > Volume 35(1); 2021 > Article |
|
Abstract
Purpose
Methods
Results
Conclusions
Notes
This study was presented at oral presentation section of 15th International Symposium in Ocular Pharmacology and Therapeutics on November 7-9, 2019 in Valencia, Spain.
Conflicts of interest
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Fig.┬Ā1
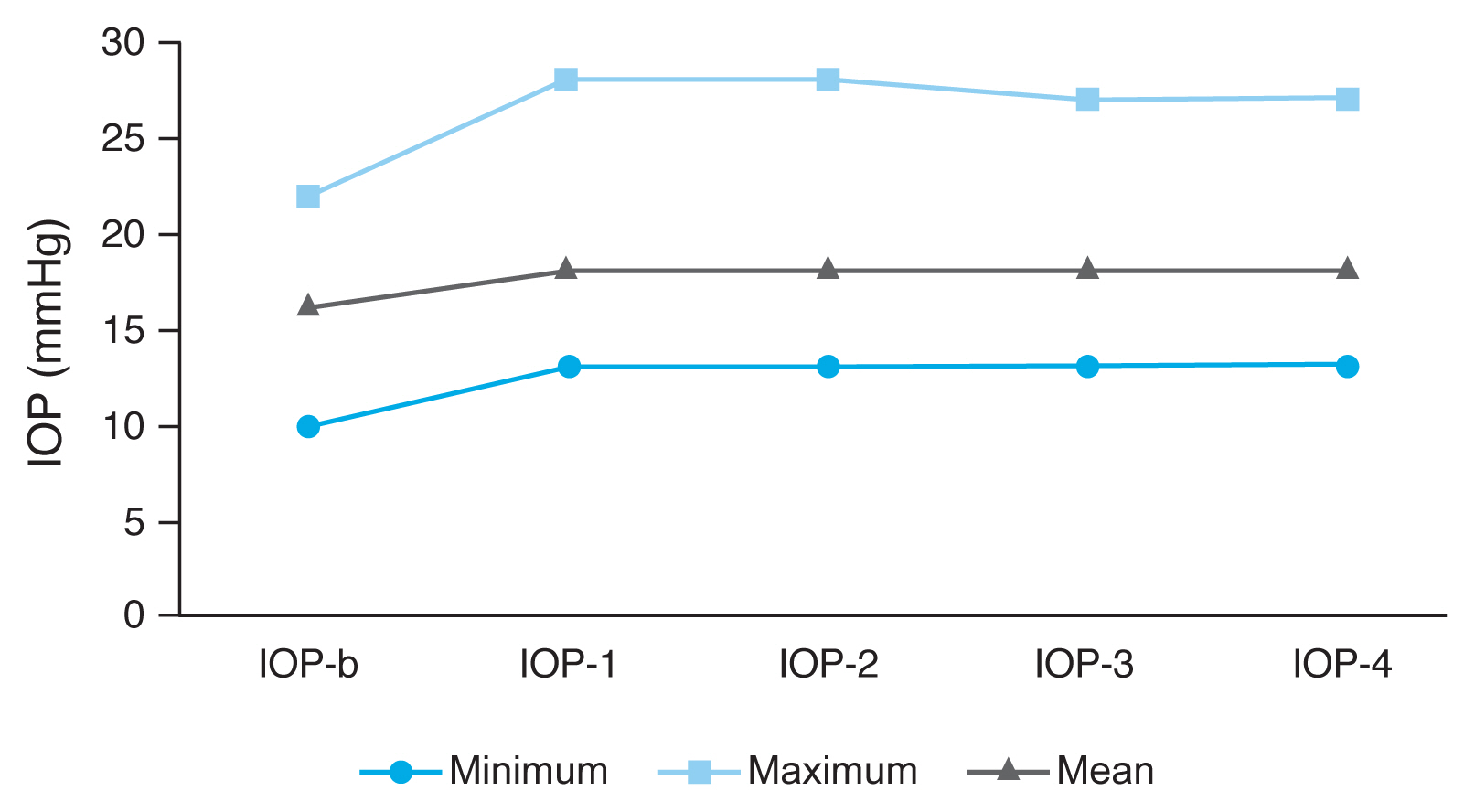
Fig.┬Ā2

Table┬Ā1
| Parameter | Value | p-value |
|---|---|---|
| IOP (mmHg) | ||
| ŌĆāIOP-b | 16.17 ┬▒ 2.71 | NA |
| ŌĆāIOP-1 | 18.15 ┬▒ 3.44 | p1 < 0.001* |
| ŌĆāIOP-2 | 18.52 ┬▒ 4.03 | p2 < 0.001* |
| ŌĆāIOP-3 | 18.39 ┬▒ 3.93 | p3 < 0.001* |
| ŌĆāIOP-4 | 17.19 ┬▒ 3.40 | p4 < 0.001* |
| ŌĆāp-valueŌĆĀ | <0.001 | |
| CCT (╬╝m) | ||
| ŌĆāCCT-b | 555.39 ┬▒ 31.90 | NA |
| ŌĆāCCT-1 | 555.00 ┬▒ 30.83 | p1 = 0.191* |
| ŌĆāCCT-2 | 555.00 ┬▒ 30.92 | p2 = 0.446* |
| ŌĆāCCT-3 | 555.50 ┬▒ 30.83 | p3 = 0.401* |
| ŌĆāCCT-4 | 555.00 ┬▒ 31.68 | p4 = 0.704* |
| ŌĆāp-value ŌĆĀ | 0.117 | |
IOP = intraocular pressure; CCT = central corneal thickness; HBOT = hyperbaric oxygen therapy; NA = not available; b = baseline value; 1 = value after 10th session of HBOT; 2 = value after 20th session of HBOT; 3 = value after 30th session of HBOT; 4 = value after 3 months of last session of HBOT; p1 = comparison of baseline and after the 10th session of HBOT; p2 = comparison of baseline and after the 20th session of HBOT; p3 = comparison of baseline and after the 30th session of HBOT; p4 = comparison of baseline and after 3 months of the last session of HBOT.
Table┬Ā2
| Parameter | Value | p-value |
|---|---|---|
| RNFL (╬╝m) | ||
| ŌĆāRNFL-IN-b | 105.86 ┬▒ 30.40 | NA |
| ŌĆāRNFL-IN-1 | 104.56 ┬▒ 29.24 | p1 = 0.124* |
| ŌĆāRNFL-IN-2 | 105.02 ┬▒ 30.63 | p2 = 0.351* |
| ŌĆāRNFL-IN-3 | 105.28 ┬▒ 30.33 | p3 = 0.606* |
| ŌĆāRNFL-IN-4 | 104.41 ┬▒ 29.39 | p4 = 0.370* |
| ŌĆāp-value ŌĆĀ | 0.096 | |
| ŌĆāRNFL-IT-b | 116.80 ┬▒ 26.46 | NA |
| ŌĆāRNFL-IT-1 | 117.17 ┬▒ 22.30 | p1 = 0.661* |
| ŌĆāRNFL-IT-2 | 118.19 ┬▒ 26.74 | p2 = 0.363* |
| ŌĆāRNFL-IT-3 | 117.52 ┬▒ 23.34 | p3 = 0.485* |
| ŌĆāRNFL-IT-4 | 116.26 ┬▒ 22.50 | p4 = 0.571* |
| ŌĆāp-value ŌĆĀ | 0.07 | |
| ŌĆāRNFL-SN-b | 100.28 ┬▒ 26.68 | NA |
| ŌĆāRNFL-SN-1 | 100.60 ┬▒ 25.26 | p1 = 0.697* |
| ŌĆāRNFL-SN-2 | 99.47 ┬▒ 27.22 | p2 = 0.477* |
| ŌĆāRNFL-SN-3 | 101.41 ┬▒ 26.49 | p3 = 0.289* |
| ŌĆāRNFL-SN-4 | 100.95 ┬▒ 26.71 | p4 = 0.455* |
| ŌĆāp-value ŌĆĀ | 0.904 | |
| ŌĆāRNFL-ST-b | 111.78 ┬▒ 25.04 | NA |
| ŌĆāRNFL-ST-1 | 112.28 ┬▒ 25.39 | p1 = 0.777* |
| ŌĆāRNFL-ST-2 | 112.45 ┬▒ 24.79 | p2 = 0.601* |
| ŌĆāRNFL-ST-3 | 112.36 ┬▒ 25.10 | p3 = 0.691* |
| ŌĆāRNFL-ST-4 | 111.73 ┬▒ 25.00 | p4 = 0.270* |
| ŌĆāp-value ŌĆĀ | 0.096 | |
| ŌĆāRNFL-T-b | 70.50 ┬▒ 10.13 | NA |
| ŌĆāRNFL-T-1 | 71.32 ┬▒ 10.28 | p1 = 0.113* |
| ŌĆāRNFL-T-2 | 69.56 ┬▒ 8.27 | p2 = 0.313* |
| ŌĆāRNFL-T-3 | 70.50 ┬▒ 9.36 | p3 = 0.818* |
| ŌĆāRNFL-T-4 | 70.26 ┬▒ 10.28 | p4 = 0.196* |
| ŌĆāp-value ŌĆĀ | 0.02 | |
| ŌĆāRNFL-N-b | 66.71 ┬▒ 11.29 | NA |
| ŌĆāRNFL-N-1 | 66.63 ┬▒ 10.25 | p1 = 0.882* |
| ŌĆāRNFL-N-2 | 66.30 ┬▒ 11.01 | p2 = 0.411* |
| ŌĆāRNFL-N-3 | 66.78 ┬▒ 10.34 | p3 = 0.775* |
| ŌĆāRNFL-N-4 | 66.45 ┬▒ 10.30 | p4 = 0.237* |
| ŌĆāp-value ŌĆĀ | 0.248 | |
RNFL = retinal nerve fiber layer; HBOT = hyperbaric oxygen therapy; NA = not available; IN = inferonasal; IT = inferotemporal; SN = superionasal; ST = superiotemporal; N = nasal; T = temporal; b = baseline measurement; 1 = measurements after 10th session of HBOT; 2 = measurements after 20th session of HBOT; 3 = measurement after 30th session of HBOT; 4 = measurements after threemonth of last session of HBOT; p1 = comparison of baseline and after the 10th session of HBOT; p2 = comparison of baseline and after the 20th session of HBOT; p3 = comparison of baseline and after the 30th session of HBOT; p4 = comparison of baseline and after 3 months of the last session of HBOT.
Table┬Ā3
| IOP value (mmHg) | HbA1c (mg/dL) | p-value* | ||
|---|---|---|---|---|
|
|
||||
| Ōēż7.0 (n = 17) | 7.0< Ōēż7.5 (n = 17) | >7.5 (n = 12) | ||
| IOP-b | 17 (10-21) | 16 (13-19) | 16 (14-21) | 0.694 |
| IOP-1 | 17 (13-22) | 17 (14-21) | 22 (14-28) | 0.029 |
| IOP-2 | 17 (13-22) | 17 (14-22) | 21.5 (14-28) | 0.018 |
| IOP-3 | 17 (13-22) | 17 (14-22) | 22.5 (17-27) | 0.049 |
| IOP-4 | 16 (12-21) | 16 (14-21) | 19 (14-21) | 0.61 |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print