 |
 |
| Korean J Ophthalmol > Volume 37(4); 2023 > Article |
|
Abstract
Purpose
Nonarteritic anterior ischemic optic neuropathy (NAION) is the second most common form of optic neuropathy. Most patients show no improvement over time. Until now, there is still no definitive therapy for NAION. The available literatures on the possible treatment of NAION are quite diverse and controversial. Neuroprotection strategies have been suggested as one of the potential treatments for NAION. This review aims to critically evaluate the literature on neuroprotective strategy for NAION.
Methods
This report was written in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. We performed a systematic literature search in Pubmed, Science Direct, Proquest, and Cochrane databases. Only neuroprotective agents that directly work in protecting neurons were included. The outcome of interest in this review is retinal ganglion cell density and apoptosis for animal studies and retinal nerve fiber layer thickness for human studies.
Results
The systematic search identified 591 studies of which 24 met the eligibility criteria, including 21 animal studies and three human studies. Only a few of the studies evaluated the same treatments, showing how diverse neuroprotector treatments are currently being evaluated as NAION treatment. From 21 animal studies, 14 studies showed significantly higher retinal ganglion cell density (1.49- to 2.81-fold) with neuroprotective treatment compared to control group. Two of three human studies in this review had also found a beneficial effect of preserving retinal nerve fiber layer thickness in NAION patients.
Nonarteritic anterior ischemic optic neuropathy (NAION) is one of the most common forms of optic neuropathy in adults, with an annual incidence of 2.3 to 10.2 per 100,000 population in the United States [1-3]. Reported incidence in the Asian population was also similar [4,5]. Patients with NAION can have a grim prognosis. Visual acuity in NAION patients can vary greatly from no light perception to 6 / 6. In untreated patients, NAION usually shows no significant improvement over time [1]. Visual outcome of NAION patients can be difficult to predict with certainty [6]. One study reported that at 12 months after onset, 61% eyes had best-corrected visual acuity ≥1.00 in logarithm of the minimum angle of resolution [7].
Until now, there is no effective treatment for NAION. Few treatment strategies have been proposed, such as optic nerve decompression surgery and steroid, but none of these treatments can provide strong evidence of efficacy to treat NAION eyes [8-10]. Considering NAION as one of the most common optic neuropathies, with its unpredictable and often poor prognosis as well as the lack of definitive treatment, studies aimed at finding a therapy for NAION are of high importance. Neuroprotection is defined as a therapeutic strategy that aims to keep retinal ganglion cells (RGC) both alive and functional [11]. In recent years, neuroprotection therapy has been suggested as a potential strategy for NAION [12,13]. This review aims to critically evaluate the currently available literature on various neuroprotective strategies for NAION.
This review was conducted using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines [14]. The study has been registered in PROSPERO (No. CRD42022333370).
This review included studies that meet the following criteria: (1) the study population included patients diagnosed with NAION or an animal model of NAION and (2) the study used a neuroprotector strategy that works directly to prevent neuronal damage. Our exclusion criteria were as follows: (1) full publication was unavailable; (2) case report or review; or (3) article not written in English. The reviewers conducted a comprehensive search on four databases, including Pubmed, Science Direct, Proquest, and Cochrane Library. The search was performed by four reviewers. The search used the following keywords: “neuro-protective,” “neuro-protector,” “neuro-enhancement,” “neuro regeneration,” “neuro rescue,” “non-arteritic ischemic optic neuropathy,” and “NAION,” including the MeSH (Medical Subject Headings) terms when available. The search terms were adapted based on the requirements of each database and hand-searching bibliographies of relevant studies were performed. Results from each database were inserted into an online-based systematic review tool, Rayyan [15]. After duplicate removal, four reviewers (BMB, EA, RWP, ADN) performed the screening process independently using this tool without knowing the decision of other reviewers. The blinding feature was turned off after the screening process was finished. Then, disagreements between reviewers were resolved through discussion. The study selection process is illustrated in Fig. 1.
Data from each study are extracted independently by four reviewers (BMB, EA, RWP, ADN). Studies were classified into two groups: animal study (Table 1) [16-36] and human study (Table 2) [37-39]. For animal studies, we extracted the data of RGC density or count as the primary outcome and apoptotic RGC count as the secondary outcome. Meanwhile, for human studies, we used the retinal nerve fiber layer (RNFL) thickness as the outcome of interest. Critical appraisal was performed by four reviewers with tools appropriate for the type of each study. Risk-of-bias assessment for animal studies was performed using the SYRCLE tool [40] and visualized using the robvis tool [41]. SYRCLE tool consists of 10 domains. Each domain was evaluated and graded as low risk, unclear, or high risk. The risk-of-bias assessment for randomized controlled trials (RCTs) was performed using RoB 2 tool [42], while the cohort study was evaluated using Newcastle-Ottawa Scale [43]. All critical appraisals were conducted independently by each reviewer. Disagreement between the four reviewers were resolved through discussion.
The article selection process following the PRISMA guideline is described in Fig. 1 [14]. The systematic search identified 591 studies of which 24 met the eligibility criteria, including 21 animal studies and three human studies. Studies in this review were from 2007 to 2021. This review included various neuroprotective treatments for NAION. Only a small number of studies evaluated the same treatment. Four studies used granulocyte colony-stimulating factor (G-CSF), two studies used omega-3 polyunsaturated fatty acids (ω-3 PUFA), and two studies used rho-kinase inhibitor of different types (E212 and Y-27632). The results of the animal studies are presented in Table 1 [16-36].
Fourteen out of 21 animal studies in this review showed significantly higher RGC density (1.49- to 2.81-fold) with neuroprotective treatment compared to the control group. Eleven out of 12 studies that evaluated the apoptotic RGC count reported significantly lower numbers (2.0- to 44.9-fold) in the treated group. Some studies that differentiated the analysis for central and mid-peripheral retina reported the same result for both analyses. The risk-of-bias assessment results for these studies is moderate. Since many studies did not describe their method in detail, the domains were dominated by “unclear” results, as pictured in Fig. 2 below.
Two of three human studies in this review had also found a beneficial effect of preserving RNFL thickness, with one of them using erythropoietin and the other citicoline. One study using levodopa found no significant difference between treatment and control group. The results of human studies are presented in Table 2 [37-39], as well as their risk-of-bias assessment results. Both RCTs in this review have a low risk of bias, meanwhile, one cohort was rated “poor quality” due to poor comparability between the treatment and control group.
To the best of our knowledge, this review is the first to assess the current literature about neuroprotective strategies in NAION. This review has assessed many treatment options that use neuroprotection for NAION eyes. Most studies in this review are animal studies, while human studies about neuroprotective strategies for NAION are still very limited. Overall, neuroprotective treatment had shown good potential as a therapy for NAION. Most studies reported a significantly higher number of RGCs and a lower number of apoptotic RGCs in the treatment group. These results indicate a good result as the neuroprotective strategies have proven to be successful in preserving RGCs.
This review has shown that the currently available literature about neuroprotective therapy for NAION is very diverse. Many studies chose neuroprotective agents that have been previously used for other diseases, from stroke and spinal cord injury to other ocular conditions such as other optic neuropathies, age-related macular degeneration, and glaucoma. While various treatments are currently being evaluated, the amount of evidence for each treatment is still limited. In this review, the most commonly used neuroprotective strategy is by using G-CSF. Two out of four studies [17,18] have reported a good result of G-CSF, with one additional study [19] that reported a good result only when G-CSF is combined with meloxicam. Two studies using ω-3 PUFA also reported good results with higher RGC count and lower apoptotic RGCs [20,21]. Two studies used rho-kinase inhibitors of different types (E212 and Y-27632) and both reported significantly higher RGC count and lower apoptotic RGCs [22,23]. Other studies in this review used different strategies from each other. Other agents who have shown good results in animal studies include puerarin, trabedonoson, intravitreal mesenchymal stem cell, astaxanthin, n-butylidenephthalide, vincamine, and PGJ2 (15-deoxy-Δ12,14-prostaglandin J2). Meanwhile, erythropoietin and citicoline had shown good results in human studies. But since these agents have shown good results in one study only, further studies are needed to support these findings. This review also included one non-pharmacological strategy using hyperbaric oxygen treatment that reported lower cell loss and fewer apoptotic RGCs in the treatment group which showed a good neuroprotective effect.
Mechanism of neuroprotection can vary between each agent, with the same goal of minimizing the damage and maximizing the recovery of the neural system after the insult to keep the RGCs alive and functional [11,44]. Although most of the mechanisms are not studied specifically in NAION, these mechanisms are used as the basis for exploring the potential of these agents for NAION eyes. Neuroprotective strategies in this review and their mechanisms are shown in Fig. 3. G-CSF can be used as a neuroprotective agent due to its anti-inflammation and antiapoptosis characteristics. G-CSF works as a cytokine that helps in recruiting extrinsic macrophages to tissues. Extrinsic macrophage activation is able to enhance remyelination, eliminate degenerated myelin, and improve axonal regeneration as well as neuronal survival. The antiapoptotic action of G-CSF works by activating various intracellular signaling pathways, mainly by the activation of phosphatidylinositol-3 kinase/protein kinase B [16,17,19]. Another neuroprotective agent, ω-3 PUFA, works also by reducing inflammation. The ω-3 PUFA is able to direct the polarization of macrophage, from the proinflammatory to the proresolving M2 phenotype, therefore reducing the level of proinflammatory cytokines. Besides its anti-inflammation effect, ω-3 PUFA also have a protective effect on the blood-optic nerve barrier by inhibiting matrix metalloproteinase production and activity. In addition, ω-3 PUFA can also promote the expressions of neurotrophins, inhibit apoptosis, and improve cell survival [20,21]. Erythropoietin also offers neuroprotective effects through multiple mechanisms, including antiapoptotic, anti-inflammatory, and antioxidant effects. It also indirectly gives neuroprotection by improving blood flow to the damaged tissue [38]. Citicoline works by inhibiting the apoptotic pathway that is induced by glutamate [45]. It can also work as a neuroprotector due to its ability to stimulate phospholipid and dopamine [46]. Meanwhile, the mechanism by which levodopa can act as a neuroprotector in NAION is still uncertain. It has been proposed that levodopa can improve visual acuity in NAION eyes by counteracting the reduced levels of dopamine in the retina, which helps in protecting the RNFL [37].
This systematic review has several limitations that needs to be taken in to account. First, many studies included in this review did not describe their method section in great detail, resulting in less-than-ideal risk-of-bias assessment results that are presented in Fig. 2. Second, the number of human studies included in this review is very limited compared to animal studies. Since an evidence-based treatment for NAION is still unavailable to date, researchers have created rodent models of NAION. This model closely imitates the human form of NAION and is used to understand the pathophysiology of this disease and to test potential treatments [47]. Although animal interventional studies are useful to explore the potential of a new treatment option, differences between animal studies and human studies should always be taken into consideration. In animal studies, the disease is often induced, intervention is given at a known time point regarding the induction, and the population is usually homogenous [40]. This also applies to animal studies of NAION. In humans, NAION is affected by many factors, different from the NAION model which is induced by a photodynamic thrombosis. Many comorbidities can be found and should be taken into account when conducting a study on humans. The time between the onset of disease to treatment can be long and undeterminable in humans. Furthermore, the amount of medication given may be insufficient for the damage. In humans, possible side effects should be considered more carefully, including possible secondary glaucoma or treatment-induced infection [48]. Considering all of these differences, it is logical that treatment that is effective in rats might not show the same effect in humans. Therefore, while animal studies are still an essential step in NAION therapy studies, translational human studies are needed to truly evaluate the efficacy and safety of neuroprotective strategies for NAION. Thirdly, this review has not provided results regarding the visual function. This review only reported the neuroprotective effect by measuring the RGC and RNFL, but it has not reported the efficacy of these neuroprotective strategies using a measure of visual function, such as visual acuity, visual field examination, or visual evoked potential. Although RGC and RNFL can portray the neuroprotective effect of each therapy, the efficacy should also be evaluated with functional assessment, since the aim of neuroprotection is not only to keep RGCs alive but also functional [11]. Lastly, this review did not include specific criteria for the duration between treatment and outcome measurement which makes the comparison between each strategy difficult. Nonetheless, this review has provided a comprehensive view of various neuroprotector treatment strategies for NAION including both animal and human studies that can be useful for future research in the pursuit of an effective treatment for NAION patients. With more research in the area, reviews with specific neuroprotective strategy might be more useful in the search for an effective therapy for NAION.
In conclusion, some neuroprotective treatments have shown encouraging results in its ability to preserve RGCs in animal model and human. However, many steps are still needed to prove the efficacy of each of these neuroprotective treatments for NAION. High-quality translational studies to study the efficacy in humans are needed, as well as studies that evaluate the safety and applicability of each of these neuroprotective treatments. Studies that evaluate the efficacy of these treatments using visual function parameters are also highly important.
Notes
References
1. Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye (Lond) 2015;29:65-79.



2. Arnold AC, Hepler RS. Fluorescein angiography in acute nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 1994;117:222-30.


3. Hattenhauer MG, Leavitt JA, Hodge DO, et al. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 1997;123:103-7.

4. Lee JY, Park KA, Oh SY. Prevalence and incidence of non-arteritic anterior ischaemic optic neuropathy in South Korea: a nationwide population-based study. Br J Ophthalmol 2018;102:936-41.


5. Xu L, Wang Y, Jonas JB. Incidence of nonarteritic anterior ischemic optic neuropathy in adult Chinese: the Beijing Eye Study. Eur J Ophthalmol 2007;17:459-60.



6. Keren S, Zanolli M, Dotan G. Visual outcome following bilateral non-arteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. BMC Ophthalmol 2017;17:155.




7. Huang HM, Wu PC, Kuo HK, et al. Natural history and visual outcome of nonarteritic anterior ischemic optic neuropathy in Southern Taiwan: a pilot study. Int Ophthalmol 2020;40:2667-76.



8. Dickersin K, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev 2015;2015:CD001538.



9. Ischemic Optic Neuropathy Decompression Trial Research Group. Ischemic optic neuropathy decompression trial: twenty-four-month update. Arch Ophthalmol 2000;118:793-8.


10. Lantos K, Domotor ZR, Farkas N, et al. Efficacy of treatments in nonarteritic ischemic optic neuropathy: a systematic review and meta-analysis. Int J Environ Res Public Health 2022;19:2718.



11. Levin LA. Retinal ganglion cells and neuroprotection for glaucoma. Surv Ophthalmol 2003;48:Suppl 1. S21-4.


12. Berry S, Lin WV, Sadaka A, Lee AG. Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye Brain 2017;9:23-8.




13. Hayreh SS. Controversies on neuroprotection therapy in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 2020;104:153-6.


14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.



15. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev 2016;5:210.




16. Slater BJ, Vilson FL, Guo Y, et al. Optic nerve inflammation and demyelination in a rodent model of nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci 2013;54:7952-61.



17. Chang CH, Huang TL, Huang SP, Tsai RK. Neuroprotective effects of recombinant human granulocyte colony-stimulating factor (G-CSF) in a rat model of anterior ischemic optic neuropathy (rAION). Exp Eye Res 2014;118:109-16.


18. Wen YT, Huang TL, Huang SP, et al. Early applications of granulocyte colony-stimulating factor (G-CSF) can stabilize the blood-optic-nerve barrier and ameliorate inflammation in a rat model of anterior ischemic optic neuropathy (rAION). Dis Model Mech 2016;9:1193-202.


19. Liu PK, Wen YT, Lin W, et al. Neuroprotective effects of low-dose G-CSF plus meloxicam in a rat model of anterior ischemic optic neuropathy. Sci Rep 2020;10:10351.




20. Georgiou T, Wen YT, Chang CH, et al. Neuroprotective effects of omega-3 polyunsaturated fatty acids in a rat model of anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci 2017;58:1603-11.


21. Huang TL, Wen YT, Ho YC, et al. Algae oil treatment protects Retinal Ganglion Cells (RGCs) via ERK signaling pathway in experimental optic nerve ischemia. Mar Drugs 2020;18:83.



22. Wen YT, Huang CW, Liu CP, et al. Inhibition of retinal ganglion cell loss by a novel ROCK inhibitor (E212) in ischemic optic nerve injury via antioxidative and anti-inflammatory actions. Invest Ophthalmol Vis Sci 2021;62:21.

23. Yi Z, Chen L, Wang X, et al. Protective effects of intravitreal injection of the rho-kinase inhibitor Y-27632 in a rodent model of nonarteritic anterior ischemic optic neuropathy (rAION). J Ophthalmol 2020;2020:1485425.




24. Le MA, Nguyen Ngo Y, Wen T, et al. Therapeutic effects of puerarin against anterior ischemic optic neuropathy through antiapoptotic and anti-inflammatory actions. Invest Ophthalmol Vis Sci 2019;60:3481-91.


25. Guo Y, Mehrabian Z, Johnson MA, et al. Topical trabodenoson is neuroprotective in a rodent model of anterior ischemic optic neuropathy (rNAION). Transl Vis Sci Technol 2019;8:47.

26. Wen YT, Ho YC, Lee YC, et al. The benefits and hazards of intravitreal Mesenchymal Stem Cell (MSC) based-therapies in the experimental ischemic optic neuropathy. Int J Mol Sci 2021;22:2117.



27. Lin WN, Kapupara K, Wen YT, et al. Haematococcus pluvialis-derived astaxanthin is a potential neuroprotective agent against optic nerve ischemia. Mar Drugs 2020;18:85.



28. Mehrabian Z, Guo Y, Weinreich D, Bernstein SL. Oligodendrocyte death, neuroinflammation, and the effects of minocycline in a rodent model of nonarteritic anterior ischemic optic neuropathy (rNAION). Mol Vis 2017;23:963-76.


29. Goldenberg-Cohen N, Dadon-Bar-El S, Hasanreisoglu M, et al. Possible neuroprotective effect of brimonidine in a mouse model of ischaemic optic neuropathy. Clin Exp Ophthalmol 2009;37:718-29.


30. Allen RS, Olsen TW, Sayeed I, et al. Progesterone treatment in two rat models of ocular ischemia. Invest Ophthalmol Vis Sci 2015;56:2880-91.



31. Chou YY, Chien JY, Ciou JW, Huang SP. The protective effects of n-butylidenephthalide on retinal ganglion cells during ischemic injury. Int J Mol Sci 2022;23:2095.



32. Li L, Su Y, Liu J, Chen C. Efficacy of Vincamine treatment in a rat model of anterior ischemic optic neuropathy. Eur J Ophthalmol 2021;31:3442-9.



33. Johnson MA, Mehrabian Z, Guo Y, et al. Anti-NOGO antibody neuroprotection in a rat model of NAION. Transl Vis Sci Technol 2021;10:12.

34. Touitou V, Johnson MA, Guo Y, et al. Sustained neuroprotection from a single intravitreal injection of PGJ2 in a rodent model of anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci 2013;54:7402-9.



35. Bernstein SL, Mehrabyan Z, Guo Y, Moianie N. Estrogen is not neuroprotective in a rodent model of optic nerve stroke. Mol Vis 2007;13:1920-5.


36. Avraham-Lubin BC, Dratviman-Storobinsky O, El SD, et al. Neuroprotective effect of hyperbaric oxygen therapy on anterior ischemic optic neuropathy. Front Neurol 2011;2:23.



37. Lyttle DP, Johnson LN, Margolin EA, Madsen RW. Levodopa as a possible treatment of visual loss in nonarteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol 2016;254:757-64.



38. Nikkhah H, Golalipour M, Doozandeh A, et al. The effect of systemic erythropoietin and oral prednisolone on recent-onset non-arteritic anterior ischemic optic neuropathy: a randomized clinical trial. Graefes Arch Clin Exp Ophthalmol 2020;258:2291-7.



39. Parisi V, Barbano L, Di Renzo A, et al. Neuroenhancement and neuroprotection by oral solution citicoline in non-arteritic ischemic optic neuropathy as a model of neurodegeneration: a randomized pilot study. PLoS One 2019;14:e0220435.



40. Hooijmans CR, Rovers MM, de Vries RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43.




41. McGuinness LA, Higgins JP. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021;12:55-61.



42. Sterne JA, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ 2019;366:l4898.

43. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] The Ottawa Hospital Research Institute; 2013 [cited 2022 May 1]. Available from: https://www.ohri.ca//programs/clinical_epidemiology/oxford.Asp.
45. Park CH, Kim YS, Noh HS, et al. Neuroprotective effect of citicoline against KA-induced neurotoxicity in the rat retina. Exp Eye Res 2005;81:350-8.


46. Rejdak R, Toczolowski J, Solski J, et al. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res 2002;34:146-9.



Fig. 1
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flowchart illustrating the process of article selection. The article selection process commenced with a comprehensive database search and hand-searching, followed by a screening procedure based on predetermined eligibility criteria. After careful screening and assessment of eligibility, a total of 24 studies were included in this review.
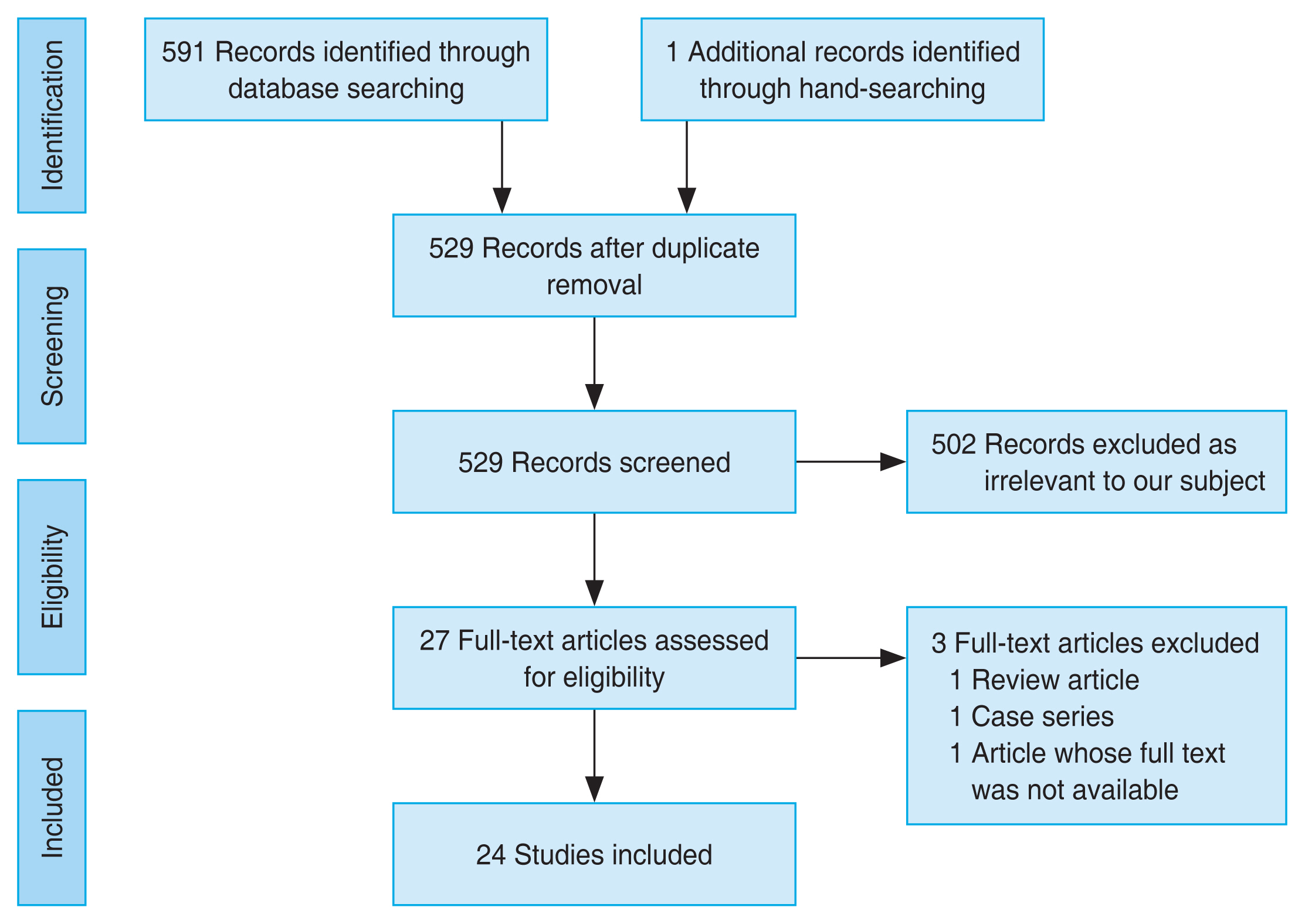
Fig. 2
Risk-of-bias assessment for all animal studies using SYRCLE [16]. While none of the studies exhibited a high risk of bias, several studies presented an “unclear” risk of bias due to lack of detailed description in the methods section. D = domain.
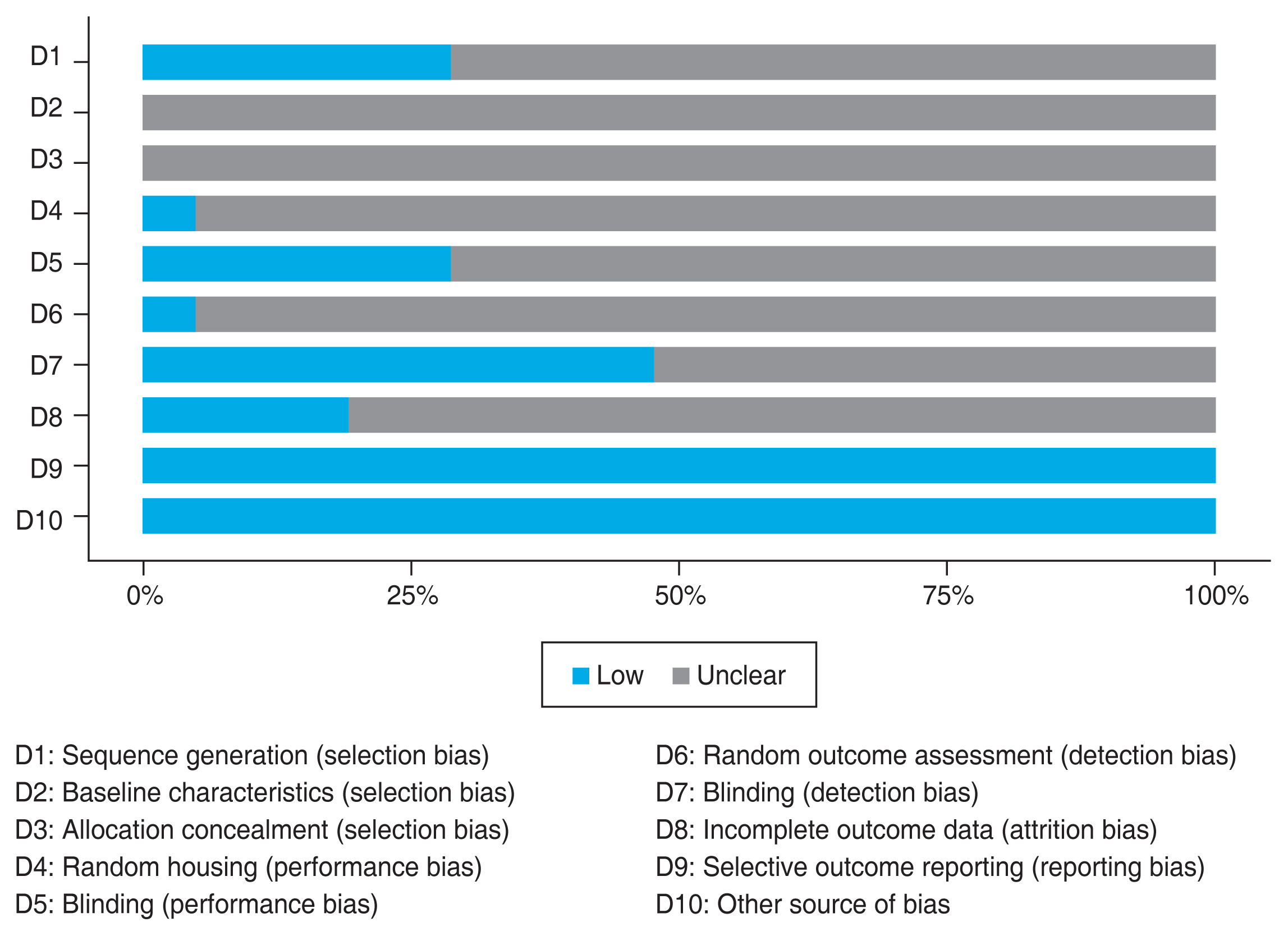
Fig. 3
Neuroprotective strategies for nonarteritic anterior ischemic optic neuropathy (NAION). Numerous neuroprotective strategies have been proposed as potential treatments for NAION. These strategies exert their effects through various mechanisms, such as antiapoptosis, stabilization of the blood-optic nerve barrier, anti-inflammation, and neurotrophic mechanisms. Some strategies demonstrate neuroprotective effects through multiple mechanisms. However, further research is necessary to explore the efficacy and underlying mechanisms of these strategies. G-CSF = granulocyte colony-stimulating factor; ω-3 PUFA = omega-3 polyunsaturated fatty acids; MSC = mesenchymal stem cell; PGJ2 = 15-deoxy-Δ12,14-prostaglandin J2.

Table 1
Results from animal studies
| Study | Intervention | RGC density (cells/mm2) | RGC apoptosis (cells/HPF) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Intervention | Control | Significance | p-value | Intervention | Control | Significance | p-value | ||
| Slater et al. [16] (2013) | GM-CSF: 2 μL of 50 ng/μL GM-CSF, intraventricularly administered 3 days postinduction | 1,079 | 1,057 | NA* | >0.91 | NA† | NA† | NA† | NA† |
| Chang et al. [17] (2014) | Recombinant human G-CSF: 100 mg/kg/day in 0.2 mL of saline, injected subcutaneously for 5 consecutive days postinduction | Central: 1,740 ± 450 | 810 ± 230 | 2.15-fold higher | 0.014 | 2.1 ± 1.0 | 8.0 ± 1.5 | 3.81-fold lower | 0.0001 |
| Mid-peripheral: 1,010 ± 410 | 360 ± 20 | 2.81-fold higher | 0.034 | ||||||
| Wen et al. [18] (2016) | G-CSF: 100 μg/kg body weight/day in 0.2 mL of saline, injected once daily subcutaneously on day 0, 1, 2, or 7 postinduction for 5 consecutive days | NA* | NA* | Day 0: 2.2-fold higher | 0.013 | NA* | NA* | Day 0: 3.7-fold lower | 0.005 |
| Day 1: 2.2-fold higher | 0.020 | Day 1: 2.0-fold lower | 0.013 | ||||||
| Liu et al. [19] (2020) | G-CSF only group: 50 μg/kg/day, administered via subcutaneous injection once daily immediately after induction for 5 consecutive days | 911.6 ± 253.5 | 587.8 ± 187.3 | Not significantly different | NA* | 10.1 ± 4.6 | 21.3 ± 2.4 | Not significantly different | NA* |
| Combination group): G-CSF (50 μg/kg/day) + meloxicam (0.125 mg/kg/day), injected subcutaneously once daily immediately after induction for 5 consecutive days + meloxicam via the oral route | 1,321.3 ± 126.5 | 2.25-fold higher | <0.005 | 4.1 ± 2.9 | NA* | 5.20-fold lower | <0.05 | ||
| Georgiou et al. [20] (2017) | ω-3 PUFA: 1 g/day eicosapentaenoic acid and 0.5 g/day docosahexaeonic acid given once daily for 10 days from day 3 before induction | Central: 1,190 ± 450 | 524 ± 201 | 2.27-fold higher | 0.03 | NA* | NA* | 2.94-fold lower | 0.007 |
| Mid-peripheral: 771 ± 201 | 374 ± 107 | 2.06-fold higher | 0.03 | ||||||
| Huang et al. [21] (2020) |
Algae oil containing docosahexaeonic acid-rich ω-3 PUFA: 1 μL/kg body weight of algae oil, via daily gavage for 7 consecutive days starting immediately after induction |
1,215 ± 504 | 506 ± 226 | 2.4-fold higher | <0.0001 | 2.3 ± 2.7 | 7.4 ± 2.4 | 3.22-fold lower | 0.001 |
| Wen et al. [22] (2021) | Rho-kinase inhibitor E212: 5 μL of E212 administered via intravitreal injection | 1,319.3 ± 160.7 | 645.1 ± 220.0 | 2.05-fold higher | <0.05 | NA* | NA* | 2.93-fold lower | <0.05 |
| Yi et al. [23] (2020) | Rho-kinase inhibitor Y-27632: 2 μL of Y-27632 administered via intravitreal injection at 1, 3, 6 days after induction | Central: 1,221 ± 109 | 676 ± 62 | 1.81-fold higher | <0.05 | NA* | NA* | Significantly lower | <0.05 |
| Mid-peripheral: 727 ± 106 | 352 ± 87 | 2.07-fold higher | <0.05 | ||||||
| Nguyen Ngo Le et al. [24] (2019) | Puerarin: 50 mg/kg/day administered via intraperitoneal injection for 3 days | 1,281.5 ± 157.8 | 786.4 ± 235.5 | 1.6-fold higher | <0.05 | 6.53 ± 2.82 | 18.21 ± 2.22 | 2.8-fold lower | <0.05 |
| Guo et al. [25] (2019) | Trabodenoson 3%: administered via ocular drops twice daily from 3 days prior to induction until 21 days postinduction | RGC loss: 27.87% ± 6.5% | RGC loss: 53.19% ± 7.08% | 1.91-fold higher loss in control group | 0.01 | NA† | NA† | NA† | NA† |
| Wen et al. [26] (2021) | Intravitreal mesenchymal stem cell: 5 μL of human Wharton’s jelly mesenchymal stem cell-suspension administered via single intravitreal injection | 48.4 ± 10.9 cells/mm | 32.4 ± 8.0 cells/mm | 1.49-fold higher | <0.05 | 0.33 ± 1.00 cells/mm | 14.83 ± 7.67 cells/mm | 44.9-fold lower | 0.0001 |
| Lin et al. [27] (2020) |
Astaxanthin: 100 mg/kg/day administered by daily gavage for 7 consecutive days Pretreatment group: 7 days before induction Posttreatment group: immediately after induction |
Pretreatment: 1,411 ± 194 | 524 ± 174 | 2.69-fold higher | <0.05 | 3.6 ± 1.9 | 12.4 ± 2.2 | 3.44-fold lower | <0.05 |
| Posttreatment: 986 ± 215 | 1.88-fold higher | <0.05 | 6.2 ± 3.3 | 2.0-fold lower | <0.05 | ||||
| Mehrabian et al. [28] (2017) | Minocycline: 33 mg/kg of minocycline hydrochloride administered via intraperitoneal injection daily for 28 days | 920.7 ± 84.50 | 893.1 ± 118.2 | NA* | 0.09 | NA† | NA† | NA† | NA† |
| Goldenberg-Cohen et al. [29] (2009) | Brimonidine tartrate ophthalmic solution in two forms: (1) 0.1 mL of topical eye drops 0.15% three times daily for 5 days after induction; or (2) intraperitoneal injection before induction at three time points | 29.6 ± 3.3 | 22.3 ± 2.7 | Not significantly different | NA* | NA† | NA† | NA† | NA† |
| Allen et al. [30] (2015) | Progesterone: 8 mg/kg of progesterone administered via intraperitoneal injection at 1-hr postinjury and then subcutaneously at 6, 24, 48, 72, 96, and 120 hr postsurgery | Progester one-treated: 47,879 ± 4,226 | 81,750 ± 3,876 | NA* | <0.001 | NA† | NA† | NA† | NA† |
| Vehicle-treat ed: 44,039 ± 4,305 | |||||||||
| Chou et al. [31] (2022) | n-butylidenephthalide: 10 mg/kg of n-butylidenephthalide administered via intraperitoneal injection for 7 days | Central: 2,172 ± 458 | 935 ± 514 | 2.32-fold higher | ≤0.001 | 3.7 ± 1.5 | 11.2 ± 2.8 | - | <0.05 |
| Mid-peripheral 1,962 ± 505 | 750 ± 451 | 2.62-fold higher | <0.0001 | ||||||
| Li et al. [32] (2021) | Vincamine: 3.15 mg/kg, dissolved in 1% NaCMC, twice a day, administered intragastrically (alone, with intravitreal PI3K inhibitor LY294002, or with intravitreal DMSO administration), days 1-28 | 1,296 ± 134 | 829 ± 199 | 1.56-fold higher | <0.05 | NA† | NA† | NA† | NA† |
| Johnson et al. [33] (2021) | Anti-Nogo receptor monoclonal antibody (11C7mAb): 2 μL of 11C7mAb antibody administered via intravitreal injection immediately after induction and 1 wk later | NA* | NA* | NA* | 0.132 | NA† | NA† | NA† | NA† |
| Touitou et al. [34] (2013) | PGJ2: 2 μL PGJ2 via intravitreal injection immediately following induction | NA* | NA* | Significantly higher | <0.02 | NA† | NA† | NA† | NA† |
| Bernstein et al. [35] (2007) | Estrogen: 50 μg/kg 17-estradiol in sesame oil injected immediately after induction | RGC loss: 65% | 70% | NA* | <0.15 | NA† | NA† | NA† | NA† |
| Avraham-Lubin et al. [36] (2011) | Hyperbaric oxygen treatment: Two 90-min sessions of 100% oxygen (2 atm), followed by one session daily for 14 days | Maximal cell loss: 8% | 27% | NA* | NA* | Day 3: 97 cells | Day 3: 180 cells | - | - |
| Day 7: 164 cells | Day 7: 600 cells | ||||||||
RGC = retinal ganglion cells; HPF = high power field; GM-CSF = granulocyte-macrophage colony-stimulating factor; NA = not applicable; G-CSF = granulocyte colony-stimulating factor; ω-3 PUFA = omega-3 polyunsaturated fatty acids; NaCMC = carboxymethylcellulose sodium; PI3K = phosphatidylinositol-3 kinase; DMSO = dimethylsulfoxide; PGJ2 = 15-deoxy-Δ12,14-prostaglandin; atm = atmosphere.
Table 2
Results from human studies
| Study | Study type | Intervention | No. of samples | RNFL thickness | Overall risk of bias | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Intervention group | Control group | p-value | |||||
| Lyttle et al. [37] (2016) | Retrospective cohort | Levodopa: 25 mg carbidopa/100 mg levodopa three times daily with meals for 12 wk | 59 | Mean reduction: 57.1% | Mean reduction: 62.5% | 0.75 | Poor quality* |
| Nikkhah et al. [38] (2020) | RCT | Erythropoietin: 10,000 units of erythropoietin twice a day for 3 days | 99 | Month 3: 110 ± 45 μm | Month 3: 119 ± 37 μm | 0.423 | Low risk† |
| Month 6: 88 ± 12 μm | Month 6: 71 ± 18 μm | 0.041‡ | |||||
| Parisi et al. [39] (2019) | RCT | Citicoline: 500 mg/day of oral solution citicoline for a 6-month period followed by 3 months of wash-out | 36 | Month 6 - baseline: 0.0552 ± 0.0529 log μ | Month 6 - baseline: 0.0510 ± 0.0421 log μ | <0.0001‡ | Low risk† |
| Month 9 - baseline: 0.0599 ± 0.0514 log μ | Month 9 - baseline: 0.0898 ± 0.0728 log μ | <0.0001‡ | |||||



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




