 |
 |
| Korean J Ophthalmol > Volume 33(4); 2019 > Article |
Abstract
Purpose
To evaluate tear film function in patients with diabetes mellitus (DM) using tear film osmolarity (TFO) measurements compared to other tear film function tests.
Methods
DM patients without any history of ocular surface disorder but with potential effects on the tear film were enrolled in this cross-sectional study. Data including dry eye symptoms, duration of DM, stage of diabetic retinopathy and blood hemoglobin A1c levels were recorded. Tear film break-up time (TBUT) and basic tear secretion (Schirmer test) were assessed. TFO was determined using the Tearlab Osmolarity System. The outcome measures were the difference between the mean values of TBUT, basic tear secretion and TFO in both the study and control groups.
Results
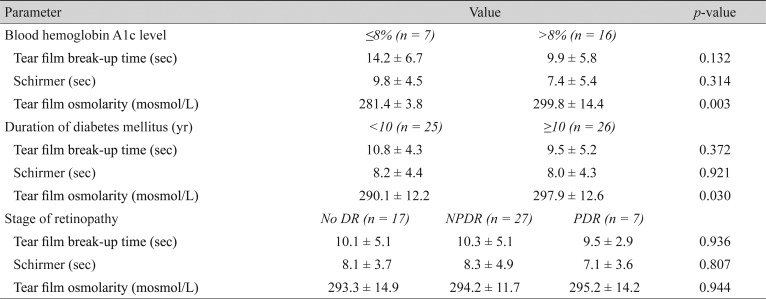
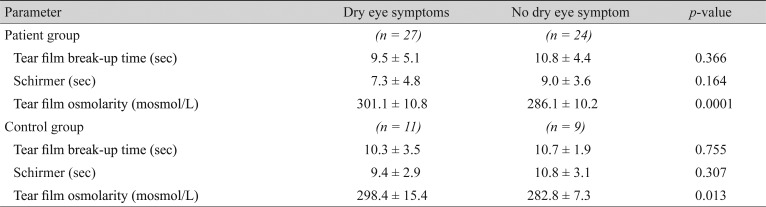
We recruited 51 DM patients and 20 control subjects with a mean age of 51.2 (range, 21 to 70) and 48.5 (range, 24 to 70) years, respectively. A total of 27 patients (53%) and 11 controls (55%) reported dry eye symptoms (p = 0.668). The mean TBUT was 10.2 ± 4.8 seconds in the study group versus 10.5 ± 2.8 seconds in controls, which was not significantly different (p = 0.747). The mean Schirmer test score was 8.1 ± 4.3 mm in the patients versus 10.1 ± 3.0 mm in the controls (p = 0.069). The mean TFO was 294.1 ± 12.9 mosmol/L in the patients versus 291.4 ± 14.5 mosmol/L in the controls (p = 0.456). It was significantly higher in patients with poor glycemic control determined by hemoglobin A1c > 8% (p = 0.003). TFO had a positive correlation with the duration of DM (p = 0.030) but not with the stage of diabetic retinopathy (p = 0.944). However, TFO showed a significant relationship with dry eye symptoms (p = 0.001).
A number of ocular complications are known to be associated with diabetes mellitus (DM) [1]. Although some diabetic ocular complications such as chronic inflammation of the eyelids, acute orbital infections, cataract and retinopathy have been discussed previously, corneal complications have only been recently studied [1]. It has been reported that 47% to 64% of patients with DM will experience a primary corneal disorder during their life time [2]. Corneal disorders related to diabetes may include epithelial fragility, microcystic edema and bleb formation, superficial punctate keratopathy, persistent epithelial defects, recurrent corneal erosions, delayed epithelial healing [3,4], decreased corneal sensitivity, decreased attachment of the epithelium to underlying layers, neurotrophic corneal ulceration, dry eye (tear film function disorders) and filamentary keratitis [5,6,7,8,9,10,11,12,13].
Tear film dysfunction and dry eye have been investigated in several studies using different methods such as tear film break-up time (TBUT), Schirmer test and pathologic evaluations [11,12,13]. Many of these studies have concluded that dry eye syndrome is more common in patients with DM than in the normal population [11,12,13]. Nevertheless, few attempts have been made to assess tear film osmolarity changes in DM patients, which is considered very sensitive for dry eye evaluation [14]. In this study, we describe tear film osmolarity changes in DM patients and compare it with the TBUT and Schirmer tests, which evaluate basic tear secretion.
This was a cross-sectional study that compared DM patients and normal individuals for pre-corneal tear film changes. The protocol was approved by the Institutional Review Board and Ethics Committee of Mashhad University of Medical Sciences (approval no. 6176) and was performed from September 2011 to 2012 in Khatam-al-Anbia Eye Hospital, Mashhad, Iran. All the patients provided an informed consent. Patients with diabetes who were referred to our center for evaluation of diabetic retinopathy were recruited. Twenty age and sex matched individuals without DM were randomly selected from refraction and retina clinics as the control group. Fasting blood sugar was checked in the control group to rule out DM. A detailed history of previous systemic and ocular diseases and medications that may have had adverse effects on tear film function was taken from each patient. None of the patients and control subjects had a history of dry eye treatment.
The exclusion criteria was as follows: history of any conjunctival and/or corneal diseases, severe meibomian gland dysfunction (MGD), previous ocular surgery and any systemic disorders that could influence tear production such as renal failure and certain endocrine diseases. Patients using anti-histamines, tricyclic anti-depressants and topical ophthalmic medications were also excluded. Those patients whose clinical examination revealed significant differences between the right and left eyelid margins were not enrolled in the study.
Demographic data and duration of diabetes were documented. The blood level of glycosylated hemoglobin (Hb) A1C was recorded for every patient. All enrolled patients underwent an ophthalmic examination, which included best-corrected visual acuity, slit-lamp examination and dilated pupil ophthalmoscopy for grading of diabetic retinopathy according to the international classification system developed by Gangaputra et al. [15]. TBUT, Schirmer test and tear film osmolarity measurements were performed before pupil dilation in order to prevent ocular surface exposure to preservatives. To measure TBUT, a fluorescein strip was introduced into the conjunctival sac with minimal conjunctival stimulation and the patient was asked to keep his or her eyes open after a few blinks. The tear film was observed with a wide slit lamp beam and cobalt blue filter. A dry spot appearance in less than 10 seconds was considered abnormal. Basic tear secretion was tested by Schirmer strips. One drop of tetracaine 0.5% (Sina Daru, Tehran, Iran) was instilled twice within a 1-minute interval and then a Schirmer strip was placed into the inferior fornix for 5 minutes and the length of the wet tape was recorded in millimeters. Wetting less than 10 millimeters was considered abnormal. Tear film osmolarity was measured with the TearLab Osmolarity System (TearLab, San Diego, CA, USA). This device only requires 0.5 mm3 of tears for osmolarity measurements, which is obtained by touching the tip of the testing card of the handle with the tear meniscus at the temporal third of the lower eyelid margin. Because the Schirmer and TBUT tests did not show any significant difference between the right and the left eyes, tear film osmolarity test was done on the right eye of the participants only. Hb A1c examination was performed at the laboratory of our clinic on the same day as the ophthalmic examinations. A value greater than 8% was considered the cutoff point of poorly controlled diabetes [16]. All of the above ocular examinations were also performed on 20 matched individuals without diabetes who met the exclusion criteria and were categorized as the control group. These subjects were selected from the refraction and retina clinics.
Statistical analysis was performed using the SPSS ver. 13 (SPSS Inc., Chicago, IL, USA). Qualitative variables were expressed as percentages, and quantitative data were expressed as mean values with standard deviation. ANOVA and t-test were used for analysis. Normal distribution of quantitative data was assessed using the Kolmogorov-Smirnov test. A p-value less than 0.05 was regarded as statistically significant.
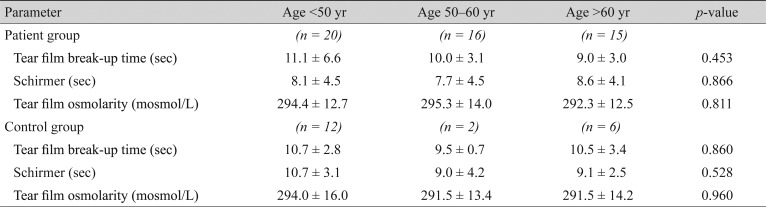
In this study, 51 DM patients and 20 controls were enrolled. Demographic data of the patients are presented in Table 1. There were 17 eyes (33.3%) with no diabetic retinopathy, 27 (53%) had non-proliferative diabetic retinopathy, and seven eyes (13.6%) had proliferative diabetic retinopathy. In total, 27 patients with DM (53%) and 11 controls (55%) reported symptoms of dry eye syndrome, such as burning, dryness and foreign body sensation. There was no statistically significant difference between the two eyes of the patients in the TBUT and Schirmer test results (t-test, p > 0.05). No age or sex predilection was observed in the ocular surface parameters (Table 2, 3). Twenty-three patients underwent a Hb A1c level examination. Of these, eight patients had good glycemic control (≤8%) and the other 15 had poor glycemic control (>8%).
The mean TBUT in the patient group was 10.2 ± 4.8 seconds (Table 1), with no significant statistical difference compared to 10.5 ± 2.8 seconds in the control group (t-test, p = 0.747). Although the patients with poorly controlled DM had a shorter TBUT compared to patients with good glycemic control, the difference was not statistically significant (p = 0.132). However, the mean TBUT in both the good and poorly controlled DM subgroups was in the normal range (Table 4). Neither the duration of DM, nor the stage of retinopathy affected the TBUT significantly (p = 0.372 and p = 0.936, respectively) (Table 4).
The mean Schirmer test value was 8.1 ± 4.3 mm in patients with diabetes versus 10.1 ± 3.0 mm in the control subjects (p = 0.069). Thirty-five percent of patients had impaired Schirmer test (values measuring less than 5 mm), whereas none of the controls had impaired test results. The Schirmer test results were better in patients with well controlled DM (Hb A1C ≤8%), compared to poor glycemic control patients; however, the difference was not statistically significant (t-test, p = 0.314) (Table 4). The Schirmer test score was neither related to the duration of DM nor to the status of diabetic retinopathy (p = 0.921 and p = 0.807, respectively).
The mean tear osmolarity values were 294.1 ± 12.9 mosmol/L in the study group versus 291.4 ± 14.6 mosmol/L in the control group (t-test, p = 0.456). Patients with poorly controlled disease (Hb A1C >8%) had a significantly higher mean tear film osmolarity than well controlled patients (299.8 vs. 281.4, respectively; p = 0.003). The mean tear film osmolarity had a significant positive correlation with the duration of DM suggesting that the longer the duration of diabetes the higher the tear film osmolarity (t-test, p = 0.03). There was no statistical correlation between the mean tear film osmolarity and the stage of diabetic retinopathy (ANOVA, p = 0.944). In both the study and control groups tear film osmolarity was higher in symptomatic compared to asymptomatic subjects (p = 0.001). Conversely, there was no relationship between the Schirmer or TBUT tests scores and dry eye symptoms in any of the study and control groups (Table 5).
Several clinical and experimental studies have reported structural, metabolic, and functional abnormalities in the conjunctiva and cornea of patients with DM and have suggested that these abnormalities may be responsible for the corneal complications of diabetes [16,17,18,19,20,21,22]. In this study, we performed tear film osmolarity measurements using the TearLab Osmolarity System and compared it with the TBUT and Schirmer tests in patients with DM. Although we found no significant difference in the tear film osmolarity between patients with DM and the normal population, the duration of DM had a significant influence on tear film osmolarity. Our study revealed a significantly higher tear film osmolarity in patients with high levels of Hb A1c (>8%) and in patients with a longer duration of DM. In a recent study, Sagdik et al. [14] showed that the patients with DM had a higher Hb A1c level than the normal population and suggested a positive correlation between tear film osmolarity and the duration of DM. However, they did not find a relationship between Hb A1c levels and tear film osmolarity. It should be mentioned that in our study, despite significant difference between tear film osmolarity in poorly controlled and well-controlled diabetes, both values were within the normal range. This result contradicts that of the Sagdik study, in which both poor and well-controlled patients had a mean tear film osmolarity higher than normal limits. Thus, we can conclude that there may be other factors that affect tear film osmolarity in patients with diabetes, such as MGD.
Our findings showed that the lower Schirmer test scores and TBUT in DM patients were not significantly different from controls. Also, the results of these tests were not affected by the status of glycemic control in the patient group. Previous studies have suggested a significant relationship between the presence/duration of DM and Schirmer test scores and TBUT [11,12,13]. As we see, our study does not confirm the results of the previous studies, which concluded that the tear film osmolarity test, Schirmer test and TBUT are impaired in patients with DM compared to the normal population. One explanation may be the fact that we excluded patients with severe MGD. Severe MGD, which is more common in DM, affects both tear film stability (evaluated by TBUT) and tear secretion (evaluated by Schirmer test) and is a known cause of tear film dysfunction. Hence, it can act as a confounding variable. Our study confirmed the Goebbels [23] and also Suzuki et al. [24] study result that showed a positive correlation between tear film osmolarity and ocular surface symptoms in cases of dry eye. Both the study and control groups maintained this relationship (p = 0.001). However, unlike their study, the TBUT and Schirmer tests failed to show a statistically significant correlation with dry eye symptoms.
Moreover, the grade of diabetic retinopathy did not have any influence on tear film osmolarity, a finding that confirms the results of previous research by Manaviat et al. [13]. Akinci et al. [12] and Manaviat et al. [13] both suggested an association between diabetes duration and dry eye using TBUT and Schirmer tests, which was not confirmed in our study. Our findings suggest that poor glycemic control, not merely having DM, is an important determinant of the tear film osmolarity and ocular surface health. Tear film osmolarity measurements have a stronger relationship with dry eye symptoms in patients with diabetes and can be used to evaluate tear film function in cases where other test results are normal. One of the limitations of our study was not being able to evaluate corneal neuropathy, which is an important part of the tear secretion reflex. The second limitation was a relatively small study population. Further studies including neuropathic assessment with a larger study population should be conducted in patients with DM to enhance our understanding of the pathogenesis of dry eye in this disease.
Acknowledgements
The authors wish to thank Dr. M. Sedaghat for his assistance with invaluable comments and for providing the Tearlab Osmolarity System.
Notes
This article was presented at the 23rd Annual Congress of the Iranian Society of Ophthalmology, 28-31 October 2013, Razi Convention Center, Tehran, Iran.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Dabbs CK, Meredith TA. Diabetic eye disease. In: Davidson JK, Clinical diabetes mellitus: a problem oriented approach. 2nd ed. New York: Thieme; 1991. p. 427-443.
2. Schultz RO, Van Horn DL, Peters MA, et al. Diabetic keratopathy. Trans Am Ophthalmol Soc 1981;79:180-199.


3. Saini JS, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol 1995;30:142-146.

4. Foulks GN, Thoft RA, Perry HD, Tolentino FI. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol 1979;97:1076-1078.


5. Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol 1977;95:2193-2196.


8. Tabatabay CA, Bumbacher M, Baumgartner B, Leuenberger PM. Reduced number of hemidesmosomes in the corneal epithelium of diabetics with proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol 1988;226:389-392.


9. Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Decreased penetration of anchoring fibrils into the diabetic stroma. A morphometric analysis. Arch Ophthalmol 1989;107:1520-1523.


10. Taylor HR, Kimsey RA. Corneal epithelial basement membrane changes in diabetes. Invest Ophthalmol Vis Sci 1981;20:548-553.

11. Modulo CM, Jorge AG, Dias AC, et al. Influence of insulin treatment on the lacrimal gland and ocular surface of diabetic rats. Endocrine 2009;36:161-168.


12. Akinci A, Cetinkaya E, Aycan Z. Dry eye syndrome in diabetic children. Eur J Ophthalmol 2007;17:873-878.


13. Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol 2008;8:10.



14. Sagdik HM, Ugurbas SH, Can M, et al. Tear film osmolarity in patients with diabetes mellitus. Ophthalmic Res 2013;50:1-5.


15. Gangaputra S, Lovato JF, Hubbard L, et al. Comparison of standardized clinical classification with fundus photograph grading for the assessment of diabetic retinopathy and diabetic macular edema severity. Retina 2013;33:1393-1399.


16. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-2559.



17. Tsubota K, Chiba K, Shimazaki J. Corneal epithelium in diabetic patients. Cornea 1991;10:156-160.


18. Shimazaki J, Tsubota K, Yoshida A, et al. Changes of corneal redox state in diabetic animal models. Cornea 1995;14:196-201.


19. Friend J, Ishii Y, Thoft RA. Corneal epithelial changes in diabetic rats. Ophthalmic Res 1982;14:269-278.


20. Chang SW, Hsu HC, Hu FR, Chen MS. Corneal autofluorescence and epithelial barrier function in diabetic patients. Ophthalmic Res 1995;27:74-79.


21. Gobbels M, Spitznas M, Oldendoerp J. Impairment of corneal epithelial barrier function in diabetics. Graefes Arch Clin Exp Ophthalmol 1989;227:142-144.


Table 1
Demographic data and ocular surface parameters of the patient and control groups and comparisons using an independent samples t-test
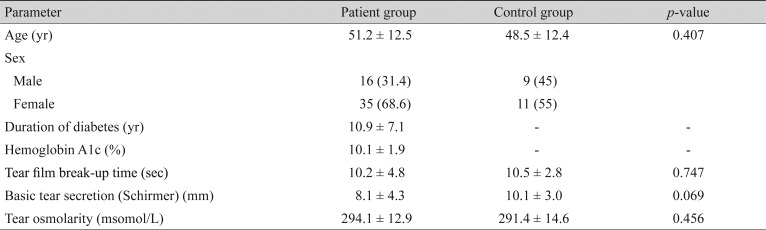
Table 2
Comparison of ocular surface parameters between males and females in the patient and control groups using an independent sample t-test

Table 3
Comparison of ocular surface parameters among age groups in the patient and control groups using the analysis of variance test
- TOOLS
-
METRICS

-
- 8 Crossref
- 0 Scopus
- 2,891 View
- 34 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



