Optic neuritis (ON), one of the most common optic neuropathies in adults [
1], is a common manifestation in multiple sclerosis (MS) or neuromyelitis optica (NMO) and can also present in isolation. NMO is considered to pathogenetically differ from MS or idiopathic ON. In NMO, necrosis and cavitation affect the gray and white matter in the spinal cord and optic nerve lesions with extensive macrophage infiltration associated with large numbers of perivascular granulocytes, eosinophils, and rare T cells [
2,
3]. Prominent vascular fibrosis and hyalinization are found in both active and inactive lesions [
2,
3,
4,
5,
6]. Compared to NMO, inflammatory demyelination is regarded as a pathologic hallmark of disease in MS. Axons are relatively preserved in MS compared to NMO. The treatment and prognosis of MS and NMO also differ somewhat. For instance, interferon betaŌĆöthe most commonly used therapy for MSŌĆöis acknowledged to be generally harmful to patients with NMO [
7,
8,
9,
10]. Reports have also suggested that natalizumab, which is also used for treating MS, could be harmful in some patients with NMO [
11,
12].
Despite pathogenic differences, discriminating among idiopathic ON, MS, and NMO is often challenging because of the similarities in clinical manifestations, especially when a unilateral ON is the only neurologic symptom. Efforts have been made to differentiate these conditions using NMO-immunoglobulin G (IgG) testing, magnetic resonance imaging (MRI), and spinal fluid analysis. The sensitivity and specificity of NMO-IgG are high, reportedly 76% and 94%, respectively [
13]. However, false negatives still occur at a rate of about 6% to 24%, and NMO-IgG testing is not routinely used for ON in the absence of suspicion for NMO. An MRI of the brain is generally recommended for every patient with ON for prognostic purposes of MS. Contrast enhancement of the optic nerve is a sensitive finding in acute ON, reported in up to 94% of cases [
14]. There has been speculation as to whether there are certain features on the optic nerve in an MRI image in patients with acute ON that suggest NMO rather than MS [
15]. The studies carried out to assist in this distinction reported a higher propensity of NMO-related ON affecting the more posterior parts of the optic nerve, including the optic chiasm [
16,
17]. The study participants included eight African Americans and nine Caucasians, and the time period of the MRI varied up to 6 weeks from the onset of ON. Considering the striking differences in the characteristics of ON between races and the acuteness of the process, we believed it was necessary to carry out an analysis of different ethnic groups with MRI performed in the acute stage and with a large sample size. In this current study, we analyzed the patterns of optic nerve enhancement in Korean patients with ON and determined if it could help to discriminate among idiopathic ON, NMO, and MS. Bilaterality itself is a strong clue for diagnosis of NMO and is one of the diagnostic criteria for NMO. Ambiguity in diagnosis of NMO mostly occurs because of the approach to unilateral idiopathic ON. We only included patients with unilateral ON as a presenting symptom in this study because we wanted to determine whether the pattern of optic nerve enhancement could help to differentiate among idiopathic ON, NMO, and MS in unilateral ON.
Materials and Methods
This comparative, observational, case series study was performed at a single center according to the tenets of the Declaration of Helsinki. The study was approved by the institutional review board of Samsung Medical Center, and written informed consent was obtained from all patients prior to enrollment. All enrolled patients had a clinical episode of unilateral ON at presentation, including visual loss and dyschromatopsia. Orbit and brain MRI within 2 weeks after the symptom onset of ON; other blood tests, such as NMO IgG, erythrocyte sedimentation rate, C-reactive protein, angiotensin-converting enzyme, antinuclear antibodies, and rapid plasma reagin test; serologic tests for toxocariasis, cat scratch disease, and Lyme disease; and a genetic test of Leber hereditary optic neuropathy were performed. Patients with idiopathic demyelinating ON were enrolled. Patients were recruited from the Neuro-ophthalmology department over a 3-year period, from March 2009 to April 2012. The diagnosis of an ON episode was based on documented findings of decreased visual acuity, visual field defect, color vision loss, relative afferent pupil defect, pain with eye movements, and a compatible fundus examination. Patients with any of the following conditions were excluded: age less than 20 years, previous ON episode within 6 months, refractive error greater than ŌłÆ6.0 diopters or +3.0 diopters (spherical equivalent), and any other ocular pathology that could affect visual functions and optical coherence tomography (OCT) measurements including glaucoma or retinal disease. NMO was diagnosed based on the NMO diagnostic guideline suggested by Wingerchuk et al. [
13] MS was diagnosed based on the McDonald criteria [
18].
All patients underwent a full ophthalmologic assessment including a visual acuity test, color vision test, slit lamp biomicroscopy, and fundus examination. Before vision testing, all subjects underwent a detailed refraction analysis. Corrected visual acuities were transformed to the logarithmic scale (logarithm of the minimal angle of resolution [logMAR]) for statistical analysis. Color vision was tested with Ishihara charts. The score was recorded as the fraction of the number of correctly identified tables over the total number of tables (e.g., 9 / 14).
Contrast sensitivity was measured with Vistech sine wave gratings (Vistech Consultants, Dayton, OH, USA) using the manufacturer's recommended testing procedure. Measurements were performed monocularly with the optimal refractive correction, natural pupil size, a chart luminance of 120 cd/m2, and a working distance of 3 m. The farthest plate on each row that was correctly seen by the subject determined contrast sensitivity. Subjects were allowed to state that they could not see any gratings. Contrast sensitivity values were converted to a logarithmic scale for statistical analysis. The visual field was tested using a Humphrey field analyzer with the 30-2 SITA-standard protocol. Only reliable visual fields were considered (Ōēż33% false positives, false negatives; fixation losses <20%), and the mean deviation was recorded. Peripapillary spectrum domain OCT (SD-OCT) retinal nerve fiber layer (RNFL) thickness measurements were performed with a Spectralis OCT (Heidelberg Engineering, Vista, CA, USA), which provides 40,000 A scans per second with 7 mm optical and 3.5 ┬Ąm digital axial resolution. The scan circle around the optic nerve is 12 degrees in diameter. Therefore, the scan circle diameter in millimeters depends on the axial length of the eye. For a typical eye length, the circle would be approximately 3.5 to 3.6 mm in diameter. The Spectralis OCT software (ver. 4.0) allows for automatic segmentation of the upper and lower borders of the RNFL to calculate the average RNFL thickness. Peripapillary RNFL thickness values are divided into four quadrants. The superior and inferior quadrants are further divided into nasal and temporal sectors.
NMO-IgG positivity was tested in all patients by cell-based indirect immunofluorescence assay at Samsung Medical Center according to the in-house protocol described in detail elsewhere [
19].
Structural imaging of the optic nerves and brain was performed in all subjects with a 3.0-tesla unit MRI system (Achieva; Philips Medical Systems, Best, The Netherlands) (
Fig. 1). The MRI protocol consisted of T1- and T2-weighted images with fat suppression with and without gadolinium-enhancement, diffusion weighted imaging (repetition time 2,500 ms, echo time 75 ms, matrix number 128 ├Ś 128, 2 b values of 0 and 1,000 s/mm
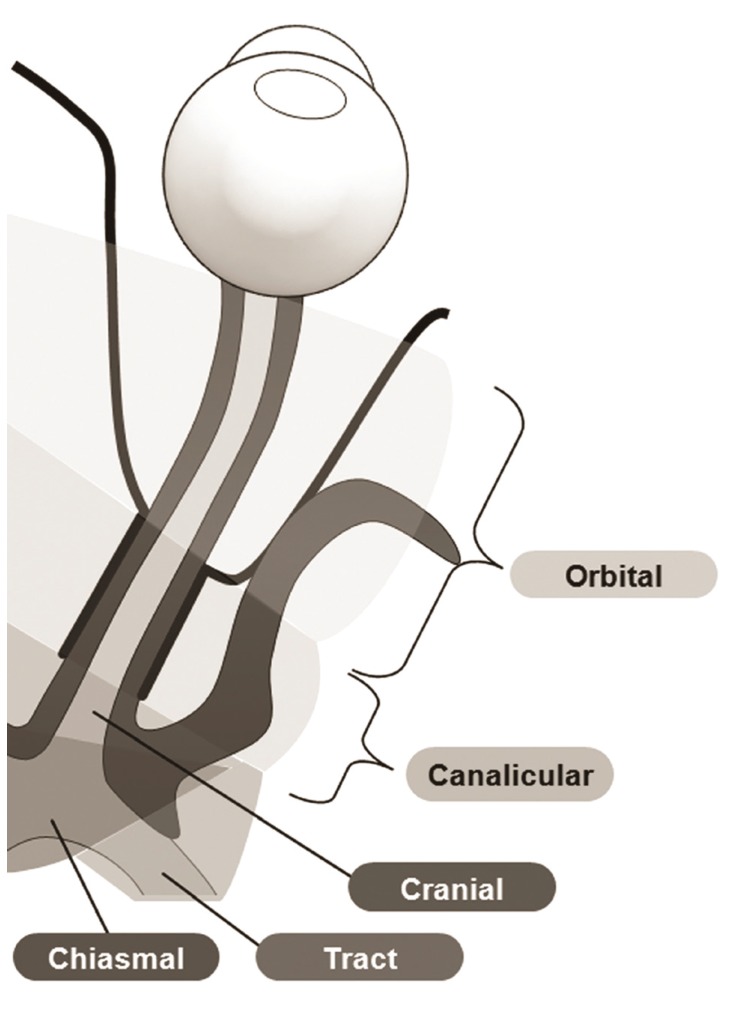
2, slice thickness 5 mm, interslice gap 2 mm, 20 axial slices, and field of view 240 mm), T2 fluid-attenuated inversion recovery (using a fast-spin echo sequence with repetition time / echo time 11,000 / 125 ms, inversion time 2,800 ms, and a 320 ├Ś 252 matrix), and gradient-echo (645 ms repetition time, 16 ms echo time, 18┬░ flip angle, 256 ├Ś 256 matrix, slice thickness 5 mm, interslice gap 2 mm, 20 axial slices, and field of view 240 mm). A single, blinded observer (JYL) calculated the length of optic nerve enhancement using T1-weighted axial images with fat suppression and gadolinium enhancement, after the lesions were identified by an experienced radiologist (HJK). The intraobserver reproducibility coefficient of variation was 4.8%. The location of enhancement was determined by segment: orbital (1), canalicular (2), prechiasmal intracranial (3), chiasmal (4), and optic tract (5) (
Fig. 2).
Statistical analyses were performed using SAS ver. 9.4 (SAS Inc., Cary, NC, USA). Correlation analysis was performed using the Spearman rank correlation two-tailed test on the final visual function including best-corrected visual acuity, color vision test score, contrast sensitivity, mean defect on visual field test, disc NFL thickness, and the diagnosis of idiopathic ON, MS, or NMO with the location of the optic nerve enhancement and length of the enhancement. The locations of optic nerve enhancement were grouped for analysis in two ways as orbital (1) vs. intracranial (2, 3, 4, and 5) and as prechiasmal (1, 2, and 3) vs. chiasmal & optic tract segments (4 and 5). The analysis of variance test and Kruskal-Wallis test were used to compare the age, time between the onset and the date of MRI examination, time between the onset and the final visual function test, logMAR visual acuity, color vision, contrast sensitivity, visual field defect, and disc NFL thickness between the ON subgroups, as appropriate. The Pearson's chi-square test was used to compare age between ON subgroups. A p-value less than 0.05 was regarded as statistically significant.
Results
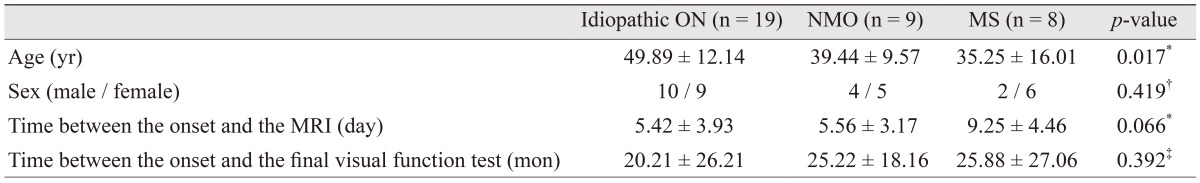
Of the 36 patients with ON who underwent orbit and brain MRI within 2 weeks, 20 (55.6%) were female. The mean age was 44.03 ┬▒ 13.77 years (mean ┬▒ standard deviation) . The mean time between the onset of symptoms and the date of the brain MRI was 6.31 ┬▒ 4.16 days. The mean time between the onset of symptoms and initial SD-OCT was 9.93 ┬▒ 25.65 months (range, 0.5 to 110 months). Most of the OCT (80.6%) was performed within 2 weeks of the onset of symptom, except that of seven patients (19.4%). The mean time between the onset of symptoms and the date of the final visual function test and SD-OCT was 22.72 ┬▒ 24.13 months. Of the total patients, 19 (52.8%) were diagnosed as idiopathic ON, 9 (25.0%) as NMO, and 8 (22.2%) as MS. The baseline characteristics of ON subgroups of NMO, MS, and idiopathic ON did not significantly differ, except age (
Table 1). Overall, 26 patients (72.2%) showed only one ON episode, while 10 patients (27.8%) showed more than two ON episodes. The mean number of ON episodes, which was 1.64 ┬▒ 1.20 in whole patients, 1.42 ┬▒ 0.90 in idiopathic ON, 2.11 ┬▒ 1.54 in NMO, and 1.63 ┬▒ 1.41 in MS, showed no significant difference among subgroups (
p = 0.372, analysis of variance).
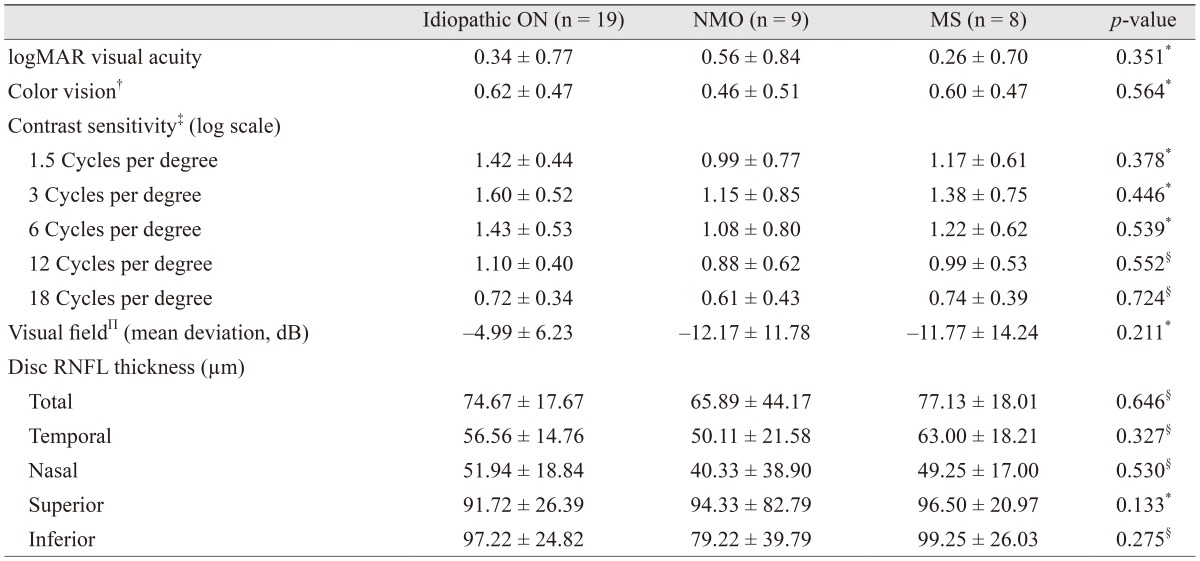
Table 2 shows the optic disc NFL thicknesses and the descriptive statistics of the functional and vision tests. There were no significant differences between the ON subgroups of NMO, MS, and idiopathic ON for any clinical parameters or for optic disc NFL thicknesses.
Enhancement of the optic nerve occurred in 21 of 36 patients (58.3%) with ON. Among 21 patients with enhancement of the optic nerve, 11 (52.4%) were diagnosed with idiopathic ON, 5 (23.8%) with NMO, and 5 (23.8%) with MS. There was no significant difference in characteristics, such as age, sex, final visual acuity, color vision, spherical equivalent, occurrence number of ON, or RNFL thickness between enhancement and non-enhancement groups (
p = 0.657, 0.446, 0.590, 0.612, 0.568, 0.141, 0.294, respectively, Mann-Whitney test). Patients with optic nerve enhancement showed a higher contrast sensitivity for a spatial frequency of 18 cycles per degree (r = 0.448,
p = 0.008; Spearman rank correlation two-tailed test). The other outcomes of final visual function, OCT parameters, and the diagnoses of idiopathic ON, MS and NMO were not significantly associated with the presence of optic nerve enhancement. The mean length of abnormal enhancement in patients with optic nerve enhancement was 19.32 ┬▒ 10.37 mm (range, 8.12 to 44.51 mm). The total length of abnormal enhancement was significantly correlated with the final contrast sensitivity for spatial frequencies of 3, 6, 12, and 18 cycles per degree (
Table 3). Patients with longer optic nerve sheath enhancement showed higher contrast sensitivity. The mean length and location of the optic nerve enhancement in ON subgroups are listed in
Table 4. The length of optic nerve enhancement was not significantly correlated with other visual functions or the diagnosis of idiopathic ON, MS, or NMO. Enhancement was limited to the orbital segment in 12 patients, to the canalicular segment in one patient, and to the optic tract segment in one patient. The other seven patients showed enhancement in two or more segments. Among these seven patients, enhancement of the optic nerve was located in the orbital and canalicular segments in four patients; the orbital, canalicular, and prechiasmal segments in one patient; and the entire segment from the orbital to the optic tract in two patients. The location of enhancement of the optic nerve was also not significantly correlated with final visual function except contrast sensitivity or the diagnosis of idiopathic ON, MS, or NMO for a spatial frequency of 6, 12, or 18 cycles per degree. The contrast sensitivity was higher when more posterior segments were involved.
Discussion
One of the notable findings in this study was that the percentage of optic nerve enhancement was quite low compared to the reported data in Western countries. Striking differences in the clinical characteristics of ON between Asian and Western countries have been previously documented: low conversion rate to MS [
20,
21,
22], higher incidence of optic disc swelling and hemorrhage, less pain [
21], and a lower prevalence of brain MRI abnormalities in ON [
22,
23,
24,
25] have been reported in Asian countries compared to figures in the West. With regard to optic nerve abnormalities on imaging, optic nerve enhancement was significantly lower in Asian countries than in Western countries, in which optic nerve enhancement was reported in up to 75% to 95% of cases [
26,
27,
28]. Optic nerve enhancement was seen in about 58% of our patients. This is higher than a previous Asian study by Wang et al. [
24], where enhancement was present in only 33%, and another earlier study by Wakakura et al. [
23], where optic nerve enhancement was reported in only 30% of cases; however, our results are lower than the 66.7% observed by Lim et al. [
29].
Because of the significant differences in the treatment and prognosis of idiopathic ON, MS, and NMO, it is crucial to differentiate them in patients with ON. Recently, Khanna et al. [
16] reported that MRI of the optic nerve could be helpful to differentiate MS and NMO in patients with ON. They reported a propensity of NMO-related ON to affect the more posterior parts of the optic nerve, including the optic chiasm. However, in our study, there were no significant correlations between diagnosis of idiopathic ON, NMO, or MS and pattern of optic nerve enhancement including the length or location of optic nerve enhancement. This could be explained by different inclusion criteria and different settings of examination. First, as ON is an acute process and the inflammation could be resolved or lessen in a short time period, we only included patients who had an MRI within 2 weeks of the first onset of symptoms. Second, although it could differ depending on the criteria applied to define NMO, simultaneous bilateral ON itself is regarded as one of the limited forms of NMO by the criteria initially suggested by Wingerchuk et al. [
30]. We only included patients with unilateral ON as a presenting symptom because our purpose was to reveal if the pattern of optic nerve enhancement could help to differentiate among idiopathic ON, NMO, and MS in unilateral ON. Racial differences could also affect the results. The striking differences in the characteristics of ON and prevalence of NMO between Western and Asian countries might reflect the different natures of the disease between these regions. Their features could differ somewhat in relation to optic nerve enhancement and the disease process.
Another interesting finding was that final contrast sensitivity was related to the pattern of optic nerve enhancement. Patients with a longer optic nerve enhancement and a more posterior location of the enhancement had higher contrast sensitivity. A previous report analyzing the correlation with visual function and the pattern of optic nerve enhancement showed that patients with involvement of the canalicular optic nerve had worse color vision and patients with longer optic nerve enhancement had worse visual acuity, contrast sensitivity, and a modestly worse visual field defect, although the correlation was weak [
28]. On the contrary, in the present study, unlike our expectation as shown in previous study, the length of the optic nerve enhancement had a positive correlation with contrast sensitivity. We believed this could be due to the distinct characteristics of Asian ON, with a high prevalence of atypical forms of ON and a related worse prognosis than typical ON in Western countries. If assuming that prominent enhancement of the optic nerve might be a characteristic reflection of the typical ON, the patients with enhancement in this study who have better contrast sensitivity could be a result of a good prognosis from a typical ON. Contrast sensitivity could be influenced by age and visual acuity, and it is proved to be a particularly practical and sensitive indicator of visual dysfunction in ON [
31].
The strength of our study was that we only used MRI images within 2 weeks of the onset of symptoms. As a consequence, the time periods of examinations were relatively narrow. The limitations of our study were that the sample size was relatively small, limiting the generalization of our conclusions, ON patients with only one episode who were diagnosed as NMO or MS were included, and we included only Korean patients. Therefore, we cannot apply these results to other populations of different races. In addition, although our previous study analyzing OCT among subgroups reported a significant difference in RNFL thickness [
32], time variation of OCT in this study resulted in a limitation in analyzing OCT data. Despite the above limitations, we found that the pattern of optic nerve enhancement was not associated with a diagnosis of idiopathic ON, NMO, or MS in Korean patients with unilateral ON. We believe that further studies that include different ethnic groups will lead to a more definitive answer on this subject.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Rodriguez M, Siva A, Cross SA, et al. Optic neuritis: a population-based study in Olmsted County, Minnesota.
Neurology 1995;45:244-250.


2. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica.
Brain 2002;125(Pt 7):1450-1461.



3. Mandler RN, Davis LE, Jeffery DR, Kornfeld M. Devic's neuromyelitis optica: a clinicopathological study of 8 patients.
Ann Neurol 1993;34:162-168.


4. Lefkowitz D, Angelo JN. Neuromyelitis optica with unusual vascular changes.
Arch Neurol 1984;41:1103-1105.


5. Stansbury FC. Neuromyelitis optica; presentation of five cases, with pathologic study, and review of literature.
Arch Ophthal 1949;42:292.


6. Scott GI. Neuromyelitis optica.
Am J Ophthalmol 1952;35:755-764.


7. Herges K, de Jong BA, Kolkowitz I, et al. Protective effect of an elastase inhibitor in a neuromyelitis optica-like disease driven by a peptide of myelin oligodendroglial glycoprotein.
Mult Scler 2012;18:398-408.



8. Krumbholz M, Faber H, Steinmeyer F, et al. Interferon-beta increases BAFF levels in multiple sclerosis: implications for B cell autoimmunity.
Brain 2008;131(Pt 6):1455-1463.


9. Palace J, Leite MI, Nairne A, Vincent A. Interferon Beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers.
Arch Neurol 2010;67:1016-1017.


10. Tanaka M, Tanaka K, Komori M. Interferon-beta(1b) treatment in neuromyelitis optica.
Eur Neurol 2009;62:167-170.


11. Barnett MH, Prineas JW, Buckland ME, et al. Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy.
Mult Scler 2012;18:108-112.


12. Kirveskari J, Bono P, Granfors K, et al. Expression of alpha4-integrins on human neutrophils.
J Leukoc Biol 2000;68:243-250.

13. Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica.
Neurology 2006;66:1485-1489.


14. Beck RW, Gal RL, Bhatti MT, et al. Visual function more than 10 years after optic neuritis: experience of the optic neuritis treatment trial.
Am J Ophthalmol 2004;137:77-83.


15. Pau D, Al Zubidi N, Yalamanchili S, et al. Optic neuritis.
Eye (Lond) 2011;25:833-842.



16. Khanna S, Sharma A, Huecker J, et al. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis.
J Neuroophthalmol 2012;32:216-220.


17. Bambach MR, Mitchell RJ. Estimating the human recovery costs of seriously injured road crash casualties.
Accid Anal Prev 2015;85:177-185.


18. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ŌĆ£McDonald CriteriaŌĆØ.
Ann Neurol 2005;58:840-846.


19. Kang ES, Min JH, Lee KH, Kim BJ. Clinical usefulness of cell-based indirect immunofluorescence assay for the detection of aquaporin-4 antibodies in neuromyelitis optica spectrum disorder.
Ann Lab Med 2012;32:331-338.



20. Isayama Y, Takahashi T, Shimoyoma T, Yamadori A. Acute optic neuritis and multiple sclerosis.
Neurology 1982;32:73-76.


21. Lim SA, Goh KY, Tow S, et al. Optic neuritis in Singapore.
Singapore Med J 2008;49:667-671.

22. Lin YC, Yen MY, Hsu WM, et al. Low conversion rate to multiple sclerosis in idiopathic optic neuritis patients in Taiwan.
Jpn J Ophthalmol 2006;50:170-175.


23. Wakakura M, Minei-Higa R, Oono S, et al. Baseline features of idiopathic optic neuritis as determined by a multicenter treatment trial in Japan: Optic Neuritis Treatment Trial Multicenter Cooperative Research Group (ONMRG).
Jpn J Ophthalmol 1999;43:127-132.


24. Wang JC, Tow S, Aung T, et al. The presentation, aetiology, management and outcome of optic neuritis in an Asian population.
Clin Exp Ophthalmol 2001;29:312-315.


25. Beck RW, Arrington J, Murtagh FR, et al. Brain magnetic resonance imaging in acute optic neuritis: Experience of the Optic Neuritis Study Group.
Arch Neurol 1993;50:841-846.


26. Hickman SJ, Miszkiel KA, Plant GT, Miller DH. The optic nerve sheath on MRI in acute optic neuritis.
Neuroradiology 2005;47:51-55.


27. Fazzone HE, Lefton DR, Kupersmith MJ. Optic neuritis: correlation of pain and magnetic resonance imaging.
Ophthalmology 2003;110:1646-1649.


28. Kupersmith MJ, Alban T, Zeiffer B, Lefton D. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance.
Brain 2002;125(Pt 4):812-822.


29. Lim SA, Sitoh YY, Chng SM, et al. Magnetic resonance imaging in acute optic neuritis in Singapore.
Ann Acad Med Singapore 2009;38:821-826.


30. Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica.
Lancet Neurol 2007;6:805-815.


31. Trobe JD, Beck RW, Moke PS, Cleary PA. Contrast sensitivity and other vision tests in the optic neuritis treatment trial.
Am J Ophthalmol 1996;121:547-553.


32. Park KA, Kim J, Oh SY. Analysis of spectral domain optical coherence tomography measurements in optic neuritis: differences in neuromyelitis optica, multiple sclerosis, isolated optic neuritis and normal healthy controls.
Acta Ophthalmol 2014;92:e57-e65.


Fig.┬Ā1
Axial views of gadolinium-enhanced T1-weighted fat-suppressed magnetic resonance imaging showing abnormal enhancement of the orbital segments of the left optic nerve.
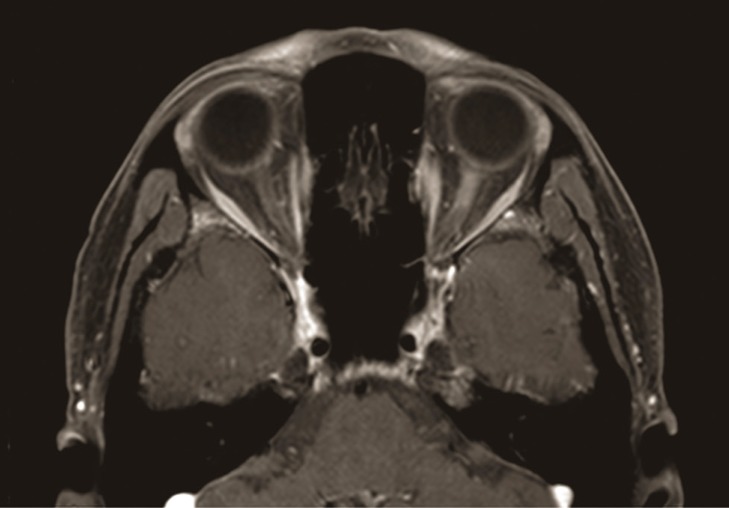
Fig.┬Ā2
Illustrations of the segments of the optic nerve used in this study.
Table┬Ā1
Demographic and clinical characteristics of study participants
Table┬Ā2
Functional and vision test results at the last visit
Table┬Ā3
Correlations of the pattern of optic nerve enhancement and the diagnosis of idiopathic ON, NMO, or MS and final visual functions

Table┬Ā4
Magnetic resonance image findings in patients with unilateral ON












 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print